FOXO1-NCOA4 Axis Contributes to Cisplatin-Induced Cochlea Spiral Ganglion Neuron Ferroptosis via Ferritinophagy
- PMID: 39206719
- PMCID: PMC11515924
- DOI: 10.1002/advs.202402671
FOXO1-NCOA4 Axis Contributes to Cisplatin-Induced Cochlea Spiral Ganglion Neuron Ferroptosis via Ferritinophagy
Abstract
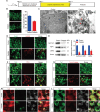
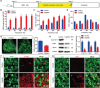
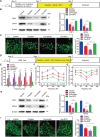
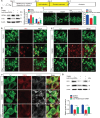
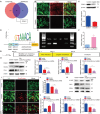
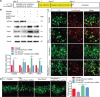
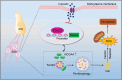
Mammalian cochlea spiral ganglion neurons (SGNs) are crucial for sound transmission, they can be damaged by chemotherapy drug cisplatin and lead to irreversible sensorineural hearing loss (SNHL), while such damage can also render cochlear implants ineffective. However, the mechanisms underlying cisplatin-induced SGNs damage and subsequent SNHL are still under debate and there is no currently effective clinical treatment. Here, this study demonstrates that ferroptosis is triggered in SGNs following exposure to cisplatin. Inhibiting ferroptosis protects against cisplatin-induced SGNs damage and hearing loss, while inducing ferroptosis intensifies these effects. Furthermore, cisplatin prompts nuclear receptor coactivator 4 (NCOA4)-mediated ferritinophagy in SGNs, while knocking down NCOA4 mitigates cisplatin-induced ferroptosis and hearing loss. Notably, the upstream regulator of NCOA4 is identified and transcription factor forkhead box O1 (FOXO1) is shown to directly suppress NCOA4 expression in SGNs. The knocking down of FOXO1 amplifies NCOA4-mediated ferritinophagy, increases ferroptosis and lipid peroxidation, while disrupting the interaction between FOXO1 and NCOA4 in NCOA4 knock out mice prevents the cisplatin-induced SGN ferroptosis and hearing loss. Collectively, this study highlights the critical role of the FOXO1-NCOA4 axis in regulating ferritinophagy and ferroptosis in cisplatin-induced SGNs damage, offering promising therapeutic targets for SNHL mitigation.
Keywords: cisplatin; ferritinophagy; ferroptosis; forkhead box transcription factor O1; hearing loss; nuclear receptor coactivator 4; spiral ganglion neuron.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
- 82271176/National Natural Science Foundation of China
- 82271172/National Natural Science Foundation of China
- 82330033/National Natural Science Foundation of China
- 82030029/National Natural Science Foundation of China
- 92149304/National Natural Science Foundation of China
- 82196821/Major Program of National Natural Science Foundation of China
- ZR2020ZD39/Major Fundamental Research Program of the Natural Science Foundation of Shandong Province
- ZR2021ZD40/Major Fundamental Research Program of the Natural Science Foundation of Shandong Province
- tsqn201909189/Taishan Scholars Program of Shandong Province
- 2021YFA1101300/National Key R&D Program of China
- 2021YFA1101800/National Key R&D Program of China
- 2020YFA0112503/National Key R&D Program of China
- BK20232007/Natural Science Foundation of Jiangsu Province
- 2021YFS0371/Science and Technology Department of Sichuan Province
- JCYJ20190814093401920/Shenzhen Science and Technology Program
- JCYJ20210324125608022/Shenzhen Science and Technology Program
- YKY-KF202201/2022 Open Project Fund of Guangdong Academy of Medical Sciences
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
