Mavacamten for Obstructive Hypertrophic Cardiomyopathy: Rationale for Clinically Guided Dose Titration to Optimize Individual Response
- PMID: 39206723
- PMCID: PMC11646538
- DOI: 10.1161/JAHA.124.033767
Mavacamten for Obstructive Hypertrophic Cardiomyopathy: Rationale for Clinically Guided Dose Titration to Optimize Individual Response
Abstract
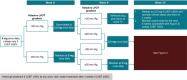
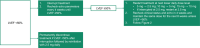
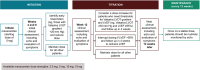
Mavacamten is the first and only cardiac myosin inhibitor approved in 5 continents for the treatment of adults with symptomatic New York Heart Association class II and III obstructive hypertrophic cardiomyopathy. An evidence-based rationale was used to develop individualized mavacamten dosing, guided by commonly used clinical parameters. Echocardiography is recommended as part of routine clinical assessment of patients with hypertrophic cardiomyopathy, and left ventricular (LV) outflow tract gradient and LV ejection fraction are parameters that can be readily assessed and monitored by echocardiography. Therefore, an echocardiography-based, clinically guided dose-titration strategy was developed to optimize patient benefit from mavacamten for the treatment of symptomatic obstructive hypertrophic cardiomyopathy while minimizing the risk of LV ejection fraction reduction. Results from clinical trials paired with extensive modeling and simulation analyses support a dose-titration and monitoring strategy based on serial echocardiographic measures of Valsalva LV outflow tract gradient and LV ejection fraction. This dosing approach allows for the identification of the lowest individualized mavacamten dose and exposure required to provide improvements in LV outflow tract obstruction, functional capacity, and symptoms. Mavacamten is primarily metabolized by CYP2C19 (cytochrome P450 2C19), and CYP2C19 metabolizer phenotype has an effect on mavacamten exposure. Therefore, this approach has also been demonstrated to provide a favorable safety profile irrespective of patients' CYP2C19 metabolizer status. The dose-titration strategy includes additional considerations for the potential onset of systolic dysfunction in the context of intercurrent illness, and for the potential of drug-drug interactions with inhibitors and substrates of cytochrome P450 enzymes. This posology is reflected in the mavacamten US prescribing information.
Keywords: echocardiogram; individualized dosing; left ventricular ejection fraction; left ventricular outflow tract gradient; mavacamten; obstructive hypertrophic cardiomyopathy.
Figures

References
-
- CAMZYOS (mavacamten) . Prescribing Information. Princeton, NJ: Bristol‐Myers Squibb Company; 2024. Accessed June 14, 2024. https://packageinserts.bms.com/pi/pi_camzyos.pdf.
-
- CAMZYOS (mavacamten) . Summary of Product Characteristics. Dublin, Ireland: Bristol‐Myers Squibb Pharma EEIG; 2023. Accessed April 22, 2024. https://www.ema.europa.eu/en/documents/product‐information/camzyos‐epar‐....
-
- Anderson RL, Trivedi DV, Sarkar SS, Henze M, Ma W, Gong H, Rogers CS, Gorham JM, Wong FL, Morck MM, et al. Deciphering the super relaxed state of human beta‐cardiac myosin and the mode of action of mavacamten from myosin molecules to muscle fibers. Proc Natl Acad Sci U S A. 2018;115:E8143–E8152. doi: 10.1073/pnas.1809540115 - DOI - PMC - PubMed
-
- Grillo MP, Erve JCL, Dick R, Driscoll JP, Haste N, Markova S, Brun P, Carlson TJ, Evanchik M. In vitro and in vivo pharmacokinetic characterization of mavacamten, a first‐in‐class small molecule allosteric modulator of beta cardiac myosin. Xenobiotica. 2019;49:718–733. doi: 10.1080/00498254.2018.1495856 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

