Clopidogrel Versus Aspirin as Monotherapy Following Dual Antiplatelet Therapy in Patients With Acute Coronary Syndrome Receiving a Drug-Eluting Stent: A Systematic Literature Review and Meta-Analysis
- PMID: 39206792
- PMCID: PMC11358762
- DOI: 10.1002/clc.24326
Clopidogrel Versus Aspirin as Monotherapy Following Dual Antiplatelet Therapy in Patients With Acute Coronary Syndrome Receiving a Drug-Eluting Stent: A Systematic Literature Review and Meta-Analysis
Abstract
Objective: This study aimed to evaluate the comparative effectiveness and safety of clopidogrel versus aspirin as monotherapy following adequate dual antiplatelet therapy (DAPT) in patients with acute coronary syndrome (ACS).
Methods: MEDLINE, Embase, and CENTRAL were searched from database inception to September 1, 2023. Randomized controlled trials (RCTs) and observational studies evaluating the effectiveness or safety of clopidogrel versus aspirin as monotherapy following DAPT in patients with ACS who received a drug-eluting stent were included. Random-effects meta-analyses were conducted to compare risks of major adverse cardiovascular events (MACE) and clinically relevant bleeding.
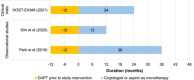
Results: Of 6242 abstracts identified, three unique studies were included: one RCT and two retrospective cohort studies. Studies included a total of 7081 post-percutaneous coronary intervention ACS patients, 4260 of whom received aspirin monotherapy and 2821 received clopidogrel monotherapy. Studies included variable proportions of patients with ST-elevation myocardial infarction (STEMI), non-STEMI, and unstable angina. From the meta-analysis, clopidogrel was associated with a 28% reduction in the risk of MACE compared with aspirin (hazard ratio [HR]: 0.72; 95% confidence interval [CI]: 0.54, 0.98), with no significant difference in clinically relevant bleeding (HR: 0.92; 95% CI: 0.68, 1.24).
Conclusion: Despite the paucity of published evidence on the effectiveness and safety of clopidogrel versus aspirin in patients with ACS post-drug-eluting stent implantation, this meta-analysis suggests that clopidogrel versus aspirin may result in a lower risk of MACE, with a similar risk of major bleeding. The present results are hypothesis-generating and further large RCTs comparing antiplatelet monotherapy options in ACS patients are warranted.
Keywords: acute coronary syndrome; aspirin; clopidogrel; dual antiplatelet therapy; meta‐analysis; single antiplatelet therapy; systematic review.
© 2024 The Author(s). Clinical Cardiology published by Wiley Periodicals, LLC.
Conflict of interest statement
Dirk Sibbing reports consulting fees and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Bayer, Daiichi Sankyo, and Sanofi, outside of the present work. Alessandro Spirito reports a research Grant from the Swiss National Science Foundation, outside of the present study. Davide Cao reports honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Terumo. Wanda Stipek and Irfan Khan are employees and shareholders at Sanofi (Bridgewater, NJ). Ellen Kasireddy and Andi Qian are employed by Evidinno Outcomes Research Inc. (Vancouver, BC, Canada), which was contracted by Sanofi to conduct this study. Roxana Mehran has received grants for institutional research from Abbott, Abiomed, Affluent Medical, Alleviant Medical, Amgen, AM‐Pharma, Arena Pharmaceuticals, AstraZeneca, Biosensors, Biotronik, Boston Scientific, Bristol Myers Squibb, CardiaWave, CeloNova, Chiesi, Cleerly Health Inc., Concept Medical, CSL Behring, Cytosorbents, Daiichi Sankyo, Element Science, Faraday Pharmaceuticals, Humacyte, Idorsia Pharmaceuticals, Janssen, Mediasphere Medical, Medtelligence, Medtronic, Novartis, OrbusNeich, Penumbra, PhaseBio, Philips, Pi‐Cardia, PLx Pharma, Protembis, ReCore Medical Inc., RenalPro, RM Global, Sanofi, Shockwave, Transverse Medical, Vivasure, and Zoll Medical. She has received consulting fees from Affluent Medical, Cordis, Henry Ford Health Cardiology, Novartis, Boehringer Ingelheim‐Lilly Partners, Ionis Pharamaceuticals, MedCon International, Novo Nordisk, Peerview Institute for Medical Education, TERUMO Europe N.V., Vectura Inc., VoxMedica, IQVIA, Radcliffe, TARSUS Cardiology, and WebMD. She is an StC Member of the Board of trustees of the American College of Cardiology, and she is an Associate Editor for JAMA. She is a member of the Scientific Advisory Board for AMA, a member of the American College of Cardiology BOT (SC Member CTR Program), and a member of the Women in Innovations Committee for the Society for Cardiovascular Angiography & Interventions. Roxana Mehran reports equity of less than 1% for Elixir Medical, Stel, and ControlRad. She is also a faculty member of the Cardiovascular Research Foundation (no fee). The remaining authors declare no conflict of interest.
Figures



References
-
- Byrne R. A., Rossello X., Coughlan J. J., et al., “2023 ESC Guidelines for the Management of Acute Coronary Syndromes,” European Heart Journal 44, no. 38 (2023): 3720–3826. - PubMed
-
- Kamran H., Jneid H., Kayani W. T., et al., “Oral Antiplatelet Therapy After Acute Coronary Syndrome: A Review,” JAMA 325, no. 15 (2021): 1545–1555. - PubMed
-
- Nicolas J., Pivato C. A., Chiarito M., Beerkens F., Cao D., and Mehran R., “Evolution of Drug‐Eluting Coronary Stents: A Back‐and‐Forth Journey From the Bench to Bedside,” Cardiovascular Research 119, no. 3 (2023): 631–646. - PubMed
-
- Levine G. N., Bates E. R., Blankenship J. C., et al., “2015 ACC/AHA/SCAI Focused Update on Primary Percutaneous Coronary Intervention for Patients With ST‐Elevation Myocardial Infarction: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of ST‐Elevation Myocardial Infarction,” Journal of the American College of Cardiology 67, no. 10 (2016): 1235–1250. - PubMed
-
- Virani S. S., Newby L. K., Arnold S. V., et al., “2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients With Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines,” Circulation 148, no. 9 (2023): e9–e119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

