Parallel electrophysiological abnormalities due to COVID-19 infection and to Alzheimer's disease and related dementia
- PMID: 39206795
- PMCID: PMC11485397
- DOI: 10.1002/alz.14089
Parallel electrophysiological abnormalities due to COVID-19 infection and to Alzheimer's disease and related dementia
Abstract
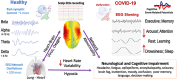
Many coronavirus disease 2019 (COVID-19) positive individuals exhibit abnormal electroencephalographic (EEG) activity reflecting "brain fog" and mild cognitive impairments even months after the acute phase of infection. Resting-state EEG abnormalities include EEG slowing (reduced alpha rhythm; increased slow waves) and epileptiform activity. An expert panel conducted a systematic review to present compelling evidence that cognitive deficits due to COVID-19 and to Alzheimer's disease and related dementia (ADRD) are driven by overlapping pathologies and neurophysiological abnormalities. EEG abnormalities seen in COVID-19 patients resemble those observed in early stages of neurodegenerative diseases, particularly ADRD. It is proposed that similar EEG abnormalities in Long COVID and ADRD are due to parallel neuroinflammation, astrocyte reactivity, hypoxia, and neurovascular injury. These neurophysiological abnormalities underpinning cognitive decline in COVID-19 can be detected by routine EEG exams. Future research will explore the value of EEG monitoring of COVID-19 patients for predicting long-term outcomes and monitoring efficacy of therapeutic interventions. HIGHLIGHTS: Abnormal intrinsic electrophysiological brain activity, such as slowing of EEG, reduced alpha wave, and epileptiform are characteristic findings in COVID-19 patients. EEG abnormalities have the potential as neural biomarkers to identify neurological complications at the early stage of the disease, to assist clinical assessment, and to assess cognitive decline risk in Long COVID patients. Similar slowing of intrinsic brain activity to that of COVID-19 patients is typically seen in patients with mild cognitive impairments, ADRD. Evidence presented supports the idea that cognitive deficits in Long COVID and ADRD are driven by overlapping neurophysiological abnormalities resulting, at least in part, from neuroinflammatory mechanisms and astrocyte reactivity. Identifying common biological mechanisms in Long COVID-19 and ADRD can highlight critical pathologies underlying brain disorders and cognitive decline. It elucidates research questions regarding cognitive EEG and mild cognitive impairment in Long COVID that have not yet been adequately investigated.
Keywords: ACE2; Alzheimer's disease and related dementia; COVID‐19; Long COVID; SARS‐CoV‐2; astrocytes; background frequency; brain fog; coronavirus and EEG; encephalopathy; inflammatory cytokine storm; long COVID; resting EEG.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare no conflicts of interest. Author disclosures are available in the Supporting information.
Figures




References
Publication types
MeSH terms
Grants and funding
- I21 RX003173/RX/RRD VA/United States
- P30 AG072946/AG/NIA NIH HHS/United States
- 1220995/ANID/FONDECYT Regular
- 1210176/ANID/FONDECYT Regular
- P01 AG078116/AG/NIA NIH HHS/United States
- 101071485/Marie Skłodowska-Curie Doctoral Networks
- R01AG057234/National Institute of Aging
- R01 AG057234/AG/NIA NIH HHS/United States
- P30AG072946/National Institute of Aging
- R56 AG063857/AG/NIA NIH HHS/United States
- 5I21RX003173/United States Department of Veterans Affairs
- R21 AG046637/AG/NIA NIH HHS/United States
- 1R21AG046637/National Institute of Aging
- P01AG078116/National Institute of Aging
- R21 AG074146/AG/NIA NIH HHS/United States
- R01 AG054484/AG/NIA NIH HHS/United States
- R01AG063857/National Institute of Aging
- HAT-07-60437/ALZ/Alzheimer's Association/United States
- R56 AG060608/AG/NIA NIH HHS/United States
- Tau Consortium
- AARF-21-848281/ALZ/Alzheimer's Association/United States
- R56AG060608/National Institute of Aging
- R01 AG063857/AG/NIA NIH HHS/United States
- 2010SH7H3F/Italian Ministry of University, Scientific and Technological Research
- MULTI-PARTNER CONSORTIUM TO EXPAND DEMENTIA RESEARCH IN LATIN AMERICA
- ACT210096/ANID/PIA/ANILLOS
- SG-20-725707/ALZ/Alzheimer's Association/United States
- 1210195/ANID/FONDECYT Regular
- P50 AG005144/AG/NIA NIH HHS/United States
- PNRR-MAD-2022-12376415/Marie Skłodowska-Curie Doctoral Networks
- CW2680521/Takeda
- 15150012/ANID/FONDAP
- H2021-MSCA-DN-2021/HORIZON 2021
- R01AG054484/National Institute of Aging
- ID22I10029/FONDEF
- United States National Institute of Health
- Global Brain Health Institute
- ID20I10152/FONDEF
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

