A Novel Antigen Design Strategy to Isolate Single-Domain Antibodies that Target Human Nav1.7 and Reduce Pain in Animal Models
- PMID: 39206821
- PMCID: PMC11516162
- DOI: 10.1002/advs.202405432
A Novel Antigen Design Strategy to Isolate Single-Domain Antibodies that Target Human Nav1.7 and Reduce Pain in Animal Models
Abstract
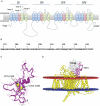
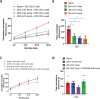
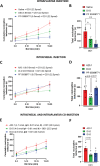
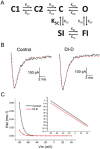
Genetic studies have identified the voltage-gated sodium channel 1.7 (Nav1.7) as pain target. Due to the ineffectiveness of small molecules and monoclonal antibodies as therapeutics for pain, single-domain antibodies (VHHs) are developed against the human Nav1.7 (hNav1.7) using a novel antigen presentation strategy. A 70 amino-acid peptide from the hNav1.7 protein is identified as a target antigen. A recombinant version of this peptide is grafted into the complementarity determining region 3 (CDR3) loop of an inert VHH in order to maintain the native 3D conformation of the peptide. This antigen is used to isolate one VHH able to i) bind hNav1.7, ii) slow the deactivation of hNav1.7, iii) reduce the ability of eliciting action potentials in nociceptors, and iv) reverse hyperalgesia in in vivo rat and mouse models. This VHH exhibits the potential to be developed as a therapeutic capable of suppressing pain. This novel antigen presentation strategy can be applied to develop biologics against other difficult targets such as ion channels, transporters and GPCRs.
Keywords: VHH; automated patch‐clamp system; epitope; mouse OD1 model; pain; rat Hargreaves model; surface plasmon resonance.
© 2024 His Majesty the King in Right of Canada. Advanced Science published by Wiley‐VCH GmbH. Reproduced with the permission of the Minister of Innovation, Science, and Economic Development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Catterall W. A., Goldin A. L., Waxman S. G., Pharmacol. Rev. 2005, 57, 397. - PubMed
-
- Bennett D. L., Clark A. J., Huang J., Waxman S. G., Dib‐Hajj S. D., Physiol. Rev. 2019, 99, 1079. - PubMed
-
- Dib‐Hajj S. D., Waxman S. G., Annu. Rev. Neurosci. 2019, 42, 87. - PubMed
-
- Dib‐Hajj S. D., Yang Y., Black J. A., Waxman S. G., Nat. Rev. Neurosci. 2013, 14, 49. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
