Design, synthesis, and biological activity evaluation of dihydromyricetin derivatives against SARS-CoV-2-Omicron virus
- PMID: 39206852
- PMCID: PMC11363738
- DOI: 10.1080/14756366.2024.2390909
Design, synthesis, and biological activity evaluation of dihydromyricetin derivatives against SARS-CoV-2-Omicron virus
Abstract
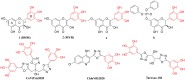
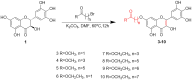
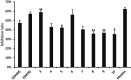
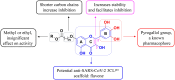
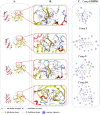
An oxidising and substituting one-pot reaction strategy has been developed to synthesise dihydromyricetin derivatives with the aim of enhancing the inhibitory activity of dihydromyricetin against SARS-CoV-2. Different ω-methoxy-ω-oxeylkyl was introduced in C7-OH site and yielded eight analogs, all of them showed good inhibitory activity against SARS-CoV-2 3CLpro with IC50 values ranging from 0.72 to 2.36 μM. In the Vero E6-cell, compound 3 has a good activity of anti-SARS-CoV-2 virus (Omicron virus BA.5) in the prevention model, with an EC50 of 15.84 μM, and so do compound 10 in the therapeutic model, with an EC50 of 11.52 μM. The results suggest that the introduction of long chain ω-oxeylkyl at C7-OH facilitate the inhibition of viral replication in the therapeutic model, which is consistent with the binding energies predicted from molecular docking conclusions. It implies that dihydromyricetin derivatives have the potential to become effective inhibitors of SARS-CoV-2 Omicron and other viruses.
Keywords: Dihydromyricetin; Omicron virus BA.5; SARS-CoV-2 3CLpro; flavone; synthesis.
Conflict of interest statement
The authors report no conflicts of interest.
Figures
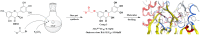
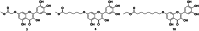
References
-
- Callaway E. What Omicron’s BA.4 and BA.5 variants mean for the pandemic. Nature. 2022;606(7916):848–849. - PubMed
-
- Tracking SARS-CoV-2 variants. Geneva (Switzerland): World Health Organization ; 2024. Jul 19 [cited 2024 Jul 26]. Available from: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
