High-throughput transposon mutagenesis in the family Enterobacteriaceae reveals core essential genes and rapid turnover of essentiality
- PMID: 39207104
- PMCID: PMC11481867
- DOI: 10.1128/mbio.01798-24
High-throughput transposon mutagenesis in the family Enterobacteriaceae reveals core essential genes and rapid turnover of essentiality
Abstract
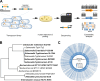
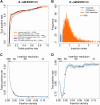
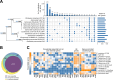
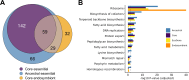
The Enterobacteriaceae are a scientifically and medically important clade of bacteria, containing the model organism Escherichia coli, as well as major human pathogens including Salmonella enterica and Klebsiella pneumoniae. Essential gene sets have been determined for several members of the Enterobacteriaceae, with the Keio E. coli single-gene deletion library often regarded as a gold standard. However, it remains unclear how gene essentiality varies between related strains and species. To investigate this, we have assembled a collection of 13 sequenced high-density transposon mutant libraries from five genera within the Enterobacteriaceae. We first assess several gene essentiality prediction approaches, investigate the effects of transposon density on essentiality prediction, and identify biases in transposon insertion sequencing data. Based on these investigations, we develop a new classifier for gene essentiality. Using this new classifier, we define a core essential genome in the Enterobacteriaceae of 201 universally essential genes. Despite the presence of a large cohort of variably essential genes, we find an absence of evidence for genus-specific essential genes. A clear example of this sporadic essentiality is given by the set of genes regulating the σE extracytoplasmic stress response, which appears to have independently acquired essentiality multiple times in the Enterobacteriaceae. Finally, we compare our essential gene sets to the natural experiment of gene loss in obligate insect endosymbionts that have emerged from within the Enterobacteriaceae. This isolates a remarkably small set of genes absolutely required for survival and identifies several instances of essential stress responses masked by redundancy in free-living bacteria.IMPORTANCEThe essential genome, that is the set of genes absolutely required to sustain life, is a core concept in genetics. Essential genes in bacteria serve as drug targets, put constraints on the engineering of biological chassis for technological or industrial purposes, and are key to constructing synthetic life. Despite decades of study, relatively little is known about how gene essentiality varies across related bacteria. In this study, we have collected gene essentiality data for 13 bacteria related to the model organism Escherichia coli, including several human pathogens, and investigated the conservation of essentiality. We find that approximately a third of the genes essential in any particular strain are non-essential in another related strain. Surprisingly, we do not find evidence for essential genes unique to specific genera; rather it appears a substantial fraction of the essential genome rapidly gains or loses essentiality during evolution. This suggests that essentiality is not an immutable characteristic but depends crucially on the genomic context. We illustrate this through a comparison of our essential genes in free-living bacteria to genes conserved in 34 insect endosymbionts with naturally reduced genomes, finding several cases where genes generally regarded as being important for specific stress responses appear to have become essential in endosymbionts due to a loss of functional redundancy in the genome.
Keywords: Citrobacter; Enterobacteriaceae; Escherichia coli; Klebsiella; Salmonella; gene essentiality; transposon mutagenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
- WT_/Wellcome Trust/United Kingdom
- bayresq.net/Bayerisches Staatsministerium für Wissenschaft und Kunst (Bavarian State Ministry of Science and the Arts)
- RGPIN-2024-04305/Canadian Government | Natural Sciences and Engineering Research Council of Canada (NSERC)
- BB/X011011/1,BB/R012504/1,BBS/E/QU/230002B,BBS/E/F/000PR10348,BBS/E/F/000PR10349/UKRI | Biotechnology and Biological Sciences Research Council (BBSRC)
- FT220100152/Australian Research Council
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
