Geriatric assessment for the practicing clinician: The why, what, and how
- PMID: 39207229
- PMCID: PMC11848937
- DOI: 10.3322/caac.21864
Geriatric assessment for the practicing clinician: The why, what, and how
Abstract
Older adults with cancer heterogeneously experience health care, treatment, and symptoms. Geriatric assessment (GA) offers a comprehensive evaluation of an older individual's health status and can predict cancer-related outcomes in individuals with solid tumors and those with hematologic malignancies. In the last decade, randomized controlled trials have demonstrated the benefits of GA and GA management (GAM), which uses GA information to provide tailored intervention strategies to address GA impairments (e.g., implementing physical therapy for impaired physical function). Multiple phase 3 clinical trials in older adults with solid tumors and hematologic malignancies have demonstrated that GAM improves treatment completion, quality of life, communication, and advance care planning while reducing treatment-related toxicity, falls, and polypharmacy. Nonetheless, implementation and uptake of GAM remain challenging. Various strategies have been proposed, including the use of GA screening tools, to identify patients most likely to benefit from GAM, the systematic engagement of the oncology workforce in the delivery of GAM, and the integration of technologies like telemedicine and mobile health to enhance the availability of GA and GAM interventions. Health inequities in minoritized groups persist, and systematic GA implementation has the potential to capture social determinants of health that are relevant to equitable care. Caregivers play an important role in cancer care and experience burden themselves. GA can guide dyadic supportive care interventions, ultimately helping both patients and caregivers achieve optimal health.
Keywords: aging; geriatric assessment; geriatric oncology; treatment‐related toxicity.
© 2024 The Author(s). CA: A Cancer Journal for Clinicians published by Wiley Periodicals LLC on behalf of American Cancer Society.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
Melody K. Schiaffino owns stock in Moderna and AstraZeneca. Grant R. Williams reports personal/consulting fees from Takeda Oncology outside the submitted work. The remaining authors disclosed no conflicts of interest.
Figures
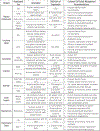
References
-
- Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod CM, ed. Evaluation of Chemotherapeutic Agents. Columbia University Press; 1949:191–205.
Publication types
MeSH terms
Grants and funding
- R00 CA237744/CA/NCI NIH HHS/United States
- K24AG056589/AG/NIA NIH HHS/United States
- R00CA237744/CA/NCI NIH HHS/United States
- Conquer Cancer Foundation American Society of Clinical Oncology-Walther Cancer Foundation Career Development Award
- K01CA276257/CA/NCI NIH HHS/United States
- K76 AG064394/AG/NIA NIH HHS/United States
- K24 AG056589/AG/NIA NIH HHS/United States
- Bristol Myers Squibb Foundation
- R03 AG073985/AG/NIA NIH HHS/United States
- R03AG073985/AG/NIA NIH HHS/United States
- DI-2020C3-21003/PCORI/Patient-Centered Outcomes Research Institute/United States
- K01 CA276257/CA/NCI NIH HHS/United States
- R0I AG077053/AG/NIA NIH HHS/United States
- K01AG068592/AG/NIA NIH HHS/United States
- R01 AG077053/AG/NIA NIH HHS/United States
- K01 AG068592/AG/NIA NIH HHS/United States
- K24 AG055693/AG/NIA NIH HHS/United States
- MRSG-18-225-01-CPPB/American Cancer Society
- R33 AG059206/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

