Detection of tumour heterogeneity in patients with advanced, metastatic castration-resistant prostate cancer on [68Ga]Ga-/[18F]F-PSMA-11/-1007, [68Ga]Ga-FAPI-46 and 2-[18F]FDG PET/CT: a pilot study
- PMID: 39207485
- PMCID: PMC11599349
- DOI: 10.1007/s00259-024-06891-8
Detection of tumour heterogeneity in patients with advanced, metastatic castration-resistant prostate cancer on [68Ga]Ga-/[18F]F-PSMA-11/-1007, [68Ga]Ga-FAPI-46 and 2-[18F]FDG PET/CT: a pilot study
Abstract
Purpose: In metastatic castration-resistant prostate cancer (mCRPC), some patients show low/absent PSMA expression in tumour lesions on positron emission tomography (PET) scans, indicating heterogeneity and heightened risk of non-response to PSMA-RLT (radioligand therapy). Imaging cancer-associated fibroblasts and glucose uptake may further characterise tumour heterogeneity in mCRPC patients. Here, we aimed to evaluate tumour heterogeneity and its potential implications for management in mCRPC patients assessed for PSMA-RLT using [68Ga]Ga-FAPI-46, 2-[18F]FDG and [68Ga]Ga-/[18F]F-PSMA-11/-1007 PET.
Material and methods: Patients with advanced, progressive mCRPC underwent clinical [68Ga]Ga-/[18F]F-PSMA-11/-1007, 2-[18F]FDG and [68Ga]Ga-FAPI-46 PET/CT to evaluate treatment with PSMA-directed RLT. Tumour detection/semiquantitative parameters were compared on a per-lesion/-region basis. Two phenotypes were defined: Criteria for the mixed phenotype were: (a) PSMA-negative findings for lymph node metastases ≥ 2.5 cm, any solid organ metastases ≥ 1.0 cm, or bone metastases with soft tissue component ≥ 1.0 cm, (b) low [68Ga]Ga-/[18F]F-PSMA-11/-1007 uptake and/or (c) balanced tumour uptake of all radioligands. The PSMA-dominant phenotype was assigned if the criteria were not met.
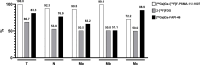
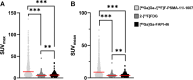
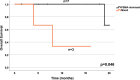
Results: In ten patients, 472 lesions were detected on all imaging modalities (miTNM regions: M1b: 327 (69.3%), M1a: 95 (20.1%), N1: 26 (5.5%), M1c: 18 (3.8%), T: 5 (1.1%) and Tr: 1 (0.2%). [68Ga]Ga-/[18F]F-PSMA-11/-1007 (n = 453 (96.0%)) demonstrates the highest detection rate, followed by [68Ga]Ga-FAPI-46 (n = 268 (56.8%))/2-[18F]FDG (n = 241 (51.1%)). Semiquantitative uptake was highest for [68Ga]Ga-/[18F]F-PSMA-11/-1007 (mean SUVmax (interquartile range): 22.7 (22.5), vs. [68Ga]Ga-FAPI-46 (7.7 (3.7)) and 2-[18F]FDG (6.8 (4.7)). Seven/three patients were retrospectively assigned to the PSMA-dominant/mixed phenotype. Median overall survival was significantly longer for patients who underwent [177Lu]Lu-PSMA-617 RLT and were retrospectively assigned to the PSMA-dominant phenotype (19.7 vs. 9.3 months).
Conclusion: Through whole-body imaging, we identify considerable inter- and intra-patient heterogeneity of mCRPC and potential imaging phenotypes. Regarding uptake and tumour detection, [68Ga]Ga-/[18F]F-PSMA-11/-1007 was superior to [68Ga]Ga-FAPI-46 and 2-[18F]FDG, while the latter two were comparable. Patients who underwent [177Lu]Lu-PSMA-617 RLT based on clinical-decision making had a longer overall survival and could be assigned to the PSMA-dominant phenotype.
Keywords: 18F-FDG; 68Ga-FAPI; MCRPC; PSMA; Prostate cancer.
© 2024. The Author(s).
Conflict of interest statement
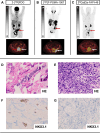
Declarations. Ethics approval: This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of University Duisburg-Essen (permits no. 19–8991-BO/20–9485-BO). Consent to participate: Informed consent was obtained from all individual participants included in the study. Consent to publish: The authors affirm that human research participants provided informed consent for publication of the images in Fig. 3 and Supplemental Fig. 1. Competing interests: K.M.P.: fees from Bayer (research funding), travel fees from IPSEN and Novartis, Clinician Scientist Stipend from the University Medicine Essen Clinician Scientist Academy (UMEA) sponsored by the faculty of medicine and Deutsche Forschungsgemeinschaft (DFG). R.M.: fees from Caelyx and Median Technologies. K.L.: consultant to Sofie Biosciences, Enlaza Therapeutics, research support from Mariana Oncology, Novartis. B.A.H.: Advisory boards for Janssen, Bayer, ABX, Lightpoint, Amgen, MSD, Pfizer, Novartis. Invited speaker for Accord, Astellas, Janssen R&D. Honoraria from Uromed. Research funding from AAA/Novartis, Bristol Myers Squibb, and German Research Foundation. Leadership roles for DKG AUO and DGU. C.K.: research fees from AAA/Novartis, Amen and Curie Therapeutics, travel fees from Amgen, Bayer and Janssen. R.H.: fees from Lilly and PharmaMar, travel fees from Lilly, Novartis and PharmaMar, Clinician Scientist Stipend from the University Medicine Essen Clinician Scientist Academy (UMEA) sponsored by the faculty of medicine and Deutsche Forschungsgemeinschaft (DFG). K.H.: personal fees: Bayer, Sofie Biosciences, SIRTEX, Adacap, Curium, Endocyte, IPSEN, Siemens Healthineers, GE Healthcare, Amgen, Novartis, ymabs, Aktis, Oncology, Pharma15; non-financial support: ABX; grants/personal fees: BTG. W.P.F.: fees from SOFIE Bioscience (research funding), Janssen (consultant, speaker), Calyx (consultant, image review), Bayer (consultant, speaker, research funding), Novartis (speaker, consultant), Telix (speaker), GE Healthcare (speaker), Eczacıbaşı Monrol (speaker), ABX (speaker), Amgen (speaker), Urotrials (speaker). All conflicts of interest were outside the submitted work.
Figures


References
-
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. - PubMed
-
- Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naïve men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survivial analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–60. - PubMed
-
- Yadav MP, Ballal S, Bal C, et al. Efficacy and Safety of 177Lu-PSMA-617 Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer Patients. Clin Nucl Med. 2020;45:19–31. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

