Comparative genomic and immunopathologic analysis of lung adenocarcinomas with and without cytology-proven malignant pleural effusions
- PMID: 39207725
- PMCID: PMC12101080
- DOI: 10.1002/cncy.22900
Comparative genomic and immunopathologic analysis of lung adenocarcinomas with and without cytology-proven malignant pleural effusions
Abstract
Background: Lung cancer complicated by malignant pleural effusions (MPEs) is associated with significantly increased morbidity and mortality, yet the mechanisms of MPE development remain poorly understood. This study sought to elucidate whether there were specific genomic alterations and/or immunologic biomarkers associated with the presence of MPEs.
Methods: Analysis of comprehensive genomic and immunologic profiling for 275 locally advanced (stage III) or advanced (stage IV) lung adenocarcinomas was subcategorized into cytology-confirmed MPE-positive (MPE+; n = 139 stage IV) and MPE-negative (MPE-; n = 30 stage III + n = 106 stage IV) groups.
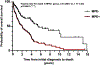
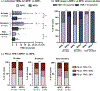
Results: Smoking frequency (p = .0001) and tumor mutational burden (p < .001) were demonstrated to be lower in the MPE+ group compared to the MPE- group. Median overall survival in the MPE+ group was shorter than in the MPE- group across all data (2.0 vs. 5.5 years; p < .0001) and for smokers (1.2 vs. 6.4 years; p < .0001). There were a number of differences at the genomic level across all cases and when stratifying by smoking status, including a higher frequency of EGFR mutations and a lower frequency of STK11 mutations in the MPE+ cohort. Finally, investigation of the comutational profiles of tumors by MPE status revealed differences in TP53- and STK11-mutant tumors between the two groups.
Conclusions: Overall, these findings imply that there are both clinical and genetic factors associated with advanced lung adenocarcinoma MPEs. Future studies of these alterations may prove important both for understanding the pathophysiology of MPE development in advanced cancer and for the earlier detection of at-risk patients.
Keywords: advanced‐stage lung adenocarcinoma; comutational profile; cytology; malignant pleural effusion; next‐generation sequencing (NGS); tumor mutational burden (TMB).
© 2024 American Cancer Society.
Conflict of interest statement
Adnan Majid reports receiving consulting fees and honoraria from Olympus America and Latin America, Boston Scientific, Cook Medical, Pulmonx, Praxis Medical, Steris, Pinnacle Biologics, and UpTo-Date, unrelated to the current work. Daniel B. Costa reports receiving consulting fees and honoraria from Takeda/Millennium Pharmaceuticals, AstraZeneca, Pfizer, Blueprint Medicines, and Janssen; institutional research support from Takeda/Millennium Pharmaceuticals, AstraZeneca, Pfizer, Merck Sharp and Dohme, Merrimack Pharmaceuticals, Bristol-Myers Squibb, Clovis Oncology, Spectrum Pharmaceuticals, Tesaro, and Daiichi Sankyo; consulting fees from Teladoc and Grand Rounds/Included Health; and royalties from Life Technologies, unrelated to the current work. Paul A. VanderLaan reports receiving consulting fees from Gala/Galvanize Therapeutics, Ruby Robotics, Intuitive Surgical, Veracyte, and Agilent Technologies, unrelated to the current work. The other author declares no conflicts of interest
Figures



References
-
- Cancer Stat Facts: Lung and Bronchus Cancer. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Accessed May 19, 2024. https://seer.cancer.gov/statfacts/html/lungb.html
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

