Identification of Immune Checkpoint Inhibitor-Induced Diabetes
- PMID: 39207773
- PMCID: PMC11362970
- DOI: 10.1001/jamaoncol.2024.3104
Identification of Immune Checkpoint Inhibitor-Induced Diabetes
Abstract
Importance: Immune checkpoint inhibitors (ICIs) have revolutionized cancer care; however, accompanying immune-related adverse events (irAEs) confer substantial morbidity and occasional mortality. Life-threatening irAEs may require permanent cessation of ICI, even in patients with positive tumor response. Therefore, it is imperative to comprehensively define the spectrum of irAEs to aid individualized decision-making around the initiation of ICI therapy.
Objective: To define incidence, risk factors, and clinical spectrum of an irreversible and life-threatening irAE: ICI-induced diabetes.
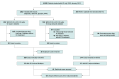
Design, setting, and participants: This cohort study, conducted at an academic integrated health care system examined 14 328 adult patients treated with ICIs, including 64 patients who developed ICI-induced diabetes, from July 2010 to January 2022. The data were analyzed from 2022 to 2023. Cases of ICI-induced diabetes were manually confirmed; detailed clinical phenotyping was performed at diagnosis and 1-year follow-up. For 862 patients, genotyping data were available, and polygenic risk for type 1 diabetes was determined.
Main outcomes and measures: For ICI-induced diabetes cases and controls, demographic characteristics, comorbidities, tumor category, and ICI category were compared. Among ICI-induced diabetes cases, markers of glycemic physiology were examined at diagnosis and 1-year follow-up. For patients with available genotyping, a published type 1 diabetes polygenic score (T1D GRS2) was calculated.
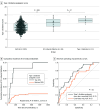
Results: Of 14 328 participants, 6571 (45.9%) were women, and the median (range) age was 66 (8-106) years. The prevalence of ICI-induced diabetes among ICI-treated patients was 0.45% (64 of 14 328), with an incidence of 124.8 per 100 000 person-years. Preexisting type 2 diabetes (odds ratio [OR], 5.91; 95% CI, 3.34-10.45) and treatment with combination ICI (OR, 2.57; 95% CI, 1.44-4.59) were significant clinical risk factors of ICI-induced diabetes. T1D GRS2 was associated with ICI-induced diabetes risk, with an OR of 4.4 (95% CI, 1.8-10.5) for patients in the top decile of T1D GRS2, demonstrating a genetic association between spontaneous autoimmunity and irAEs. Patients with ICI-induced diabetes were in 3 distinct phenotypic categories based on autoantibodies and residual pancreatic function, with varying severity of initial presentation.
Conclusions and relevance: The results of this analysis of 14 328 ICI-treated patients followed up from ICI initiation determined the incidence, risk factors and clinical spectrum of ICI-induced diabetes. Widespread implementation of this approach across organ-specific irAEs may enhance diagnosis and management of these conditions, and this becomes especially pertinent as ICI treatment rapidly expands to treat a wide spectrum of cancers and is used at earlier stages of treatment.
Conflict of interest statement
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

