B cell-based therapy produces antibodies that inhibit glioblastoma growth
- PMID: 39207859
- PMCID: PMC11473152
- DOI: 10.1172/JCI177384
B cell-based therapy produces antibodies that inhibit glioblastoma growth
Abstract
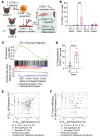
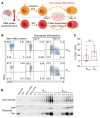
Glioblastoma (GBM) is a highly aggressive and malignant brain tumor with limited therapeutic options and a poor prognosis. Despite current treatments, the invasive nature of GBM often leads to recurrence. A promising alternative strategy is to harness the potential of the immune system against tumor cells. Our previous data showed that the BVax (B cell-based vaccine) can induce therapeutic responses in preclinical models of GBM. In this study, we aimed to characterize the antigenic reactivity of BVax-derived Abs and evaluate their therapeutic potential. We performed immunoproteomics and functional assays in murine models and samples from patients with GBM. Our investigations revealed that BVax distributed throughout the GBM tumor microenvironment and then differentiated into Ab-producing plasmablasts. Proteomics analyses indicated that the Abs produced by BVax had unique reactivity, predominantly targeting factors associated with cell motility and the extracellular matrix. Crucially, these Abs inhibited critical processes such as GBM cell migration and invasion. These findings provide valuable insights into the therapeutic potential of BVax-derived Abs for patients with GBM, pointing toward a novel direction for GBM immunotherapy.
Keywords: Brain cancer; Cancer immunotherapy; Extracellular matrix; Immunology; Oncology.
Figures






References
MeSH terms
Substances
Grants and funding
- R01 CA120813/CA/NCI NIH HHS/United States
- R01 NS120547/NS/NINDS NIH HHS/United States
- R37 CA276851/CA/NCI NIH HHS/United States
- R01 CA279686/CA/NCI NIH HHS/United States
- P30 CA015083/CA/NCI NIH HHS/United States
- R35 CA197725/CA/NCI NIH HHS/United States
- S10 OD025194/OD/NIH HHS/United States
- P41 GM108569/GM/NIGMS NIH HHS/United States
- T32 GM145408/GM/NIGMS NIH HHS/United States
- P30 CA060553/CA/NCI NIH HHS/United States
- R01 NS115955/NS/NINDS NIH HHS/United States
- P50 CA221747/CA/NCI NIH HHS/United States
- R37 CA258426/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

