West Nile virus triggers intestinal dysmotility via T cell-mediated enteric nervous system injury
- PMID: 39207863
- PMCID: PMC11527448
- DOI: 10.1172/JCI181421
West Nile virus triggers intestinal dysmotility via T cell-mediated enteric nervous system injury
Abstract
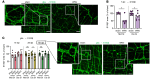
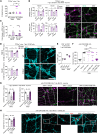
Intestinal dysmotility syndromes have been epidemiologically associated with several antecedent bacterial and viral infections. To model this phenotype, we previously infected mice with the neurotropic flavivirus West Nile virus (WNV) and demonstrated intestinal transit defects. Here, we found that within 1 week of WNV infection, enteric neurons and glia became damaged, resulting in sustained reductions of neuronal cells and their networks of connecting fibers. Using cell-depleting antibodies, adoptive transfer experiments, and mice lacking specific immune cells or immune functions, we show that infiltrating WNV-specific CD4+ and CD8+ T cells damaged the enteric nervous system (ENS) and glia, which led to intestinal dysmotility; these T cells used multiple and redundant effector molecules including perforin and Fas ligand. In comparison, WNV-triggered ENS injury and intestinal dysmotility appeared to not require infiltrating monocytes, and damage may have been limited by resident muscularis macrophages. Overall, our experiments support a model in which antigen-specific T cell subsets and their effector molecules responding to WNV infection direct immune pathology against enteric neurons and supporting glia that results in intestinal dysmotility.
Keywords: Fas signaling; Gastroenterology; Infectious disease; Neurological disorders; T cells.
Conflict of interest statement
Figures





Comment in
- Experimental West Nile virus infection provides lessons for recovery from enteric neuropathies
Similar articles
-
Experimental West Nile virus infection provides lessons for recovery from enteric neuropathies.J Clin Invest. 2024 Nov 1;134(21):e185865. doi: 10.1172/JCI185865. J Clin Invest. 2024. PMID: 39484722 Free PMC article.
-
The Interferon-Stimulated Gene Ifitm3 Restricts West Nile Virus Infection and Pathogenesis.J Virol. 2016 Aug 26;90(18):8212-25. doi: 10.1128/JVI.00581-16. Print 2016 Sep 15. J Virol. 2016. PMID: 27384652 Free PMC article.
-
CD8+ T cells use TRAIL to restrict West Nile virus pathogenesis by controlling infection in neurons.J Virol. 2012 Sep;86(17):8937-48. doi: 10.1128/JVI.00673-12. Epub 2012 Jun 27. J Virol. 2012. PMID: 22740407 Free PMC article.
-
CD8 and CD4 T cells in west nile virus immunity and pathogenesis.Viruses. 2013 Oct 22;5(10):2573-84. doi: 10.3390/v5102573. Viruses. 2013. PMID: 24153060 Free PMC article. Review.
-
Immunology of West Nile Virus Infection and the Role of Alpha-Synuclein as a Viral Restriction Factor.Viral Immunol. 2019 Jan/Feb;32(1):38-47. doi: 10.1089/vim.2018.0075. Epub 2018 Sep 15. Viral Immunol. 2019. PMID: 30222521 Review.
Cited by
-
Innate immune sensing of rotavirus by intestinal epithelial cells leads to diarrhea.Cell Host Microbe. 2025 Mar 12;33(3):408-419.e8. doi: 10.1016/j.chom.2025.02.005. Epub 2025 Mar 3. Cell Host Microbe. 2025. PMID: 40037352
-
Ex vivo study of neuroinvasive and neurotropic viruses: what is current and what is next.FEMS Microbiol Rev. 2025 Jan 14;49:fuaf024. doi: 10.1093/femsre/fuaf024. FEMS Microbiol Rev. 2025. PMID: 40498321 Free PMC article. Review.
-
Short-chain fatty acids mediate enteric and central nervous system homeostasis in Parkinson's disease: Innovative therapies and their translation.Neural Regen Res. 2026 Mar 1;21(3):938-956. doi: 10.4103/NRR.NRR-D-24-01265. Epub 2025 Apr 29. Neural Regen Res. 2026. PMID: 40313087 Free PMC article.
-
Experimental West Nile virus infection provides lessons for recovery from enteric neuropathies.J Clin Invest. 2024 Nov 1;134(21):e185865. doi: 10.1172/JCI185865. J Clin Invest. 2024. PMID: 39484722 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

