Increased Urine Excretion of Neutrophil Granule Cargo in Active Proliferative Lupus Nephritis
- PMID: 39207891
- PMCID: PMC11371349
- DOI: 10.34067/KID.0000000000000491
Increased Urine Excretion of Neutrophil Granule Cargo in Active Proliferative Lupus Nephritis
Abstract
Key Points:
Neutrophil degranulation participates in glomerular injury in proliferative lupus nephritis.
Urine excretion of neutrophil granule proteins is a potential diagnostic for proliferative lupus nephritis.
Background: Lupus nephritis (LN) occurs in more than half of patients with systemic lupus erythematosus, but the cellular and molecular events that contribute to LN are not clearly defined. We reported previously that neutrophil degranulation participates in glomerular injury in mouse models of acute LN. This study tests the hypothesis that glomerular recruitment and subsequent activation of neutrophils result in urine excretion of neutrophil granule constituents that are predictive of glomerular inflammation in proliferative LN.
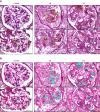
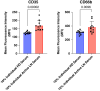
Methods: Urine and serum levels of 11 neutrophil granule proteins were measured by antibody-based array in patients with proliferative LN and healthy donors (HDs), and the results were confirmed by ELISA. Glomerular neutrophil accumulation was assessed in biopsies of patients with LN who contributed urine for granule cargo quantitation and normal kidney tissue by microscopy. Degranulation was measured by flow cytometry in neutrophils isolated from patients with LN and HD controls by cell surface granule markers CD63 (azurophilic), CC66b (specific), and CD35 (secretory). Nonparametric statistical analyses were performed and corrected for multiple comparisons.
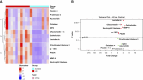
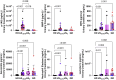
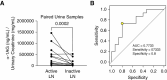
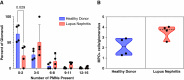
Results: Eight granule proteins (myeloperoxidase, neutrophil elastase, azurocidin, olfactomedin-4, lactoferrin, alpha-1-acid glycoprotein 1, matrix metalloproteinase 9, and cathelicidin) were significantly elevated in urine from patients with active proliferative LN by array and/or ELISA, whereas only neutrophil elastase was increased in LN serum. Urine excretion of alpha-1-acid glycoprotein 1 declined in patients who achieved remission. The majority of LN glomeruli contained ≥3 neutrophils. Basal levels of specific granule markers were increased in neutrophils from patients with LN compared with HD controls. Serum from patients with active LN stimulated specific and secretory, but not azurophilic granule, release by HD neutrophils.
Conclusions: Circulating neutrophils in patients with LN are primed for enhanced degranulation. Glomerular recruitment of those primed neutrophils leads to release and urine excretion of neutrophil granule cargo that serves as a urine marker of active glomerular inflammation in proliferative LN.
Conflict of interest statement
Disclosure forms, as provided by each author, are available with the online version of the article at
Figures




Similar articles
-
[Antigens of antineutrophil cytoplasmic autoantibodies in recognizing a novel neutrophil granule antigen in lupus nephritis and its association with photosensitivity and oral ulceration].Zhonghua Nei Ke Za Zhi. 2002 Nov;41(11):766-8. Zhonghua Nei Ke Za Zhi. 2002. PMID: 12485525 Chinese.
-
Prospective validation of a novel renal activity index of lupus nephritis.Lupus. 2017 Aug;26(9):927-936. doi: 10.1177/0961203316684212. Epub 2016 Dec 19. Lupus. 2017. PMID: 28361601 Free PMC article.
-
Increased excretions of beta2-microglobulin, IL-6, and IL-8 and decreased excretion of Tamm-Horsfall glycoprotein in urine of patients with active lupus nephritis.Nephron. 2000 Jul;85(3):207-14. doi: 10.1159/000045663. Nephron. 2000. PMID: 10867535
-
Neutrophils in lupus nephritis.Curr Opin Rheumatol. 2019 Mar;31(2):193-200. doi: 10.1097/BOR.0000000000000577. Curr Opin Rheumatol. 2019. PMID: 30540580 Review.
-
The Role of Autophagy in Lupus Nephritis.Int J Mol Sci. 2015 Oct 22;16(10):25154-67. doi: 10.3390/ijms161025154. Int J Mol Sci. 2015. PMID: 26506346 Free PMC article. Review.
Cited by
-
Serine proteases and protease-activated receptors signaling in the kidney.Am J Physiol Cell Physiol. 2025 Jul 1;329(1):C107-C117. doi: 10.1152/ajpcell.00143.2025. Epub 2025 Apr 17. Am J Physiol Cell Physiol. 2025. PMID: 40244049 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

