Regiodivergent Gold-Catalyzed Rearrangement-Addition Reactions of Sulfenylated Propargylic Carboxylates with Indoles
- PMID: 39207898
- PMCID: PMC11406574
- DOI: 10.1021/acs.orglett.4c02853
Regiodivergent Gold-Catalyzed Rearrangement-Addition Reactions of Sulfenylated Propargylic Carboxylates with Indoles
Abstract
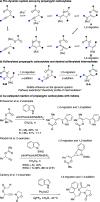
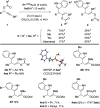
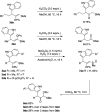
Sulfenylated propargylic carboxylates were introduced to investigate the influence of sulfur substitution in gold-catalyzed alkyne activation pathways. Regiodivergent gold-catalyzed rearrangement and indole capture reactions proceed under mild conditions to give functionalized indole products bearing sulfenylated (Z)-enol carboxylate motifs. Pathways involving both 1,2- and 1,3-carboxylate migrations are achieved selectively, with indole being added in a 1,4 relationship to the sulfenyl group in each case. High levels of selectivity are influenced by the catalyst system, counterion, and carboxylate group.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

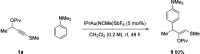
Similar articles
-
Synthesis of diverse indole-containing scaffolds by gold(I)-catalyzed tandem reactions of 3-propargylindoles initiated by 1,2-indole migrations: scope and computational studies.Chemistry. 2010 Aug 23;16(32):9818-28. doi: 10.1002/chem.201001162. Chemistry. 2010. PMID: 20645356
-
Selectivity, Speciation, and Substrate Control in the Gold-Catalyzed Coupling of Indoles and Alkynes.Organometallics. 2022 Feb 28;41(4):497-507. doi: 10.1021/acs.organomet.2c00035. Epub 2022 Feb 10. Organometallics. 2022. PMID: 35431397 Free PMC article.
-
Atom-efficient gold(I)-chloride-catalyzed synthesis of α-sulfenylated carbonyl compounds from propargylic alcohols and aryl thiols: substrate scope and experimental and theoretical mechanistic investigation.Chemistry. 2013 Dec 23;19(52):17939-50. doi: 10.1002/chem.201302485. Epub 2013 Nov 22. Chemistry. 2013. PMID: 24272980 Free PMC article.
-
Metal-catalyzed regiodivergent organic reactions.Chem Soc Rev. 2019 Aug 12;48(16):4515-4618. doi: 10.1039/c8cs00872h. Chem Soc Rev. 2019. PMID: 31282495 Review.
-
Recent developments in the metal-catalyzed reactions of metallocarbenoids from propargylic esters.Chemistry. 2007;13(5):1350-7. doi: 10.1002/chem.200601522. Chemistry. 2007. PMID: 17200921 Review.
References
-
- Campeau D.; Leon Rayo D. F.; Mansour A.; Muratov K.; Gagosz F. Gold-Catalyzed Reactions of Specially Activated Alkynes, Allenes, and Alkenes. Chem. Rev. 2021, 121, 8756–8867. 10.1021/acs.chemrev.0c00788. - DOI - PubMed
- Davies P. W. Gold-Catalyzed Annulations with Nucleophilic Nitrenoids Enabled by Heteroatom-Substituted Alkynes. Chem. Rec. 2021, 21, 3964–3977. 10.1002/tcr.202100205. - DOI - PubMed
- Shandilya S.; Protim Gogoi M.; Dutta S.; Sahoo A. K. Gold-Catalyzed Transformation of Ynamides. Chem. Rec. 2021, 21, 4123–4149. 10.1002/tcr.202100159. - DOI - PubMed
-
- Jiang X.Sulfur Chemistry; Springer International Publishing: Cham, Switzerland, 2018; Vol. 376.
-
-
α-Selective:
- Ye X.; Wang J.; Ding S.; Hosseyni S.; Wojtas L.; Akhmedov N. G.; Shi X. Investigations on Gold-Catalyzed Thioalkyne Activation Toward Facile Synthesis of Ketene Dithioacetals. Chem. - Eur. J. 2017, 23, 10506–10510. 10.1002/chem.201702710. - DOI - PMC - PubMed
- Bai Y.-B.; Luo Z.; Wang Y.; Gao J.-M.; Zhang L. Au-Catalyzed Intermolecular [2 + 2] Cycloadditions between Chloroalkynes and Unactivated Alkenes. J. Am. Chem. Soc. 2018, 140, 5860–5865. 10.1021/jacs.8b01813. - DOI - PMC - PubMed
- Wang J.; Zhang S.; Xu C.; Wojtas L.; Akhmedov N. G.; Chen H.; Shi X. Highly Efficient and Stereoselective Thioallylation of Alkynes: Possible Gold Redox Catalysis with No Need for a Strong Oxidant. Angew. Chem., Int. Ed. 2018, 57, 6915–6920. 10.1002/anie.201802540. - DOI - PMC - PubMed
- Sharma P.; Singh R. R.; Giri S. S.; Chen L.-Y.; Cheng M.-J.; Liu R.-S. Gold-Catalyzed Oxidation of Thioalkynes To Form Phenylthio Ketene Derivatives via a Noncarbene Route. Org. Lett. 2019, 21, 5475–5479. 10.1021/acs.orglett.9b01768. - DOI - PubMed
-
-
-
β-Selective:
- Sekar P.; Gupta A.; English L. E.; Rabbitt C. E.; Male L.; Jupp A. R.; Davies P. W. Regiodivergent Synthesis of 4- and 5-Sulfenyl Oxazoles from Alkynyl Thioethers. Chem. - Eur. J. 2024, 30, e20240146510.1002/chem.202401465. - DOI - PubMed
- Simm P. E.; Sekar P.; Richardson J.; Davies P. W. Gold(I)-Catalyzed Synthesis of 3-Sulfenyl Pyrroles and Indoles by a Regioselective Annulation of Alkynyl Thioethers. ACS Catal. 2021, 11, 6357–6362. 10.1021/acscatal.1c01457. - DOI - PMC - PubMed
- Reddy R. J.; Ball-Jones M. P.; Davies P. W. Alkynyl Thioethers in Gold-Catalyzed Annulations To Form Oxazoles. Angew. Chem., Int. Ed. 2017, 56, 13310–13313. 10.1002/anie.201706850. - DOI - PMC - PubMed
-
LinkOut - more resources
Full Text Sources

