Opposing actions of co-released GABA and neurotensin on the activity of preoptic neurons and on body temperature
- PMID: 39207910
- PMCID: PMC11361704
- DOI: 10.7554/eLife.98677
Opposing actions of co-released GABA and neurotensin on the activity of preoptic neurons and on body temperature
Abstract
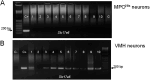
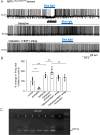
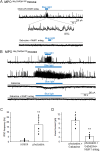
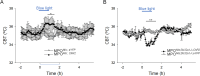
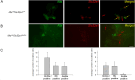
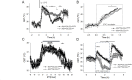
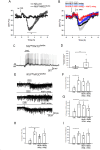
Neurotensin (Nts) is a neuropeptide acting as a neuromodulator in the brain. Pharmacological studies have identified Nts as a potent hypothermic agent. The medial preoptic area, a region that plays an important role in the control of thermoregulation, contains a high density of neurotensinergic neurons and Nts receptors. The conditions in which neurotensinergic neurons play a role in thermoregulation are not known. In this study, optogenetic stimulation of preoptic Nts neurons induced a small hyperthermia. In vitro, optogenetic stimulation of preoptic Nts neurons resulted in synaptic release of GABA and net inhibition of the preoptic pituitary adenylate cyclase-activating polypeptide (Adcyap1) neurons firing activity. GABA-A receptor antagonist or genetic deletion of Slc32a1 (VGAT) in Nts neurons unmasked also an excitatory effect that was blocked by a Nts receptor 1 antagonist. Stimulation of preoptic Nts neurons lacking Slc32a1 resulted in excitation of Adcyap1 neurons and hypothermia. Mice lacking Slc32a1 expression in Nts neurons presented changes in the fever response and in the responses to heat or cold exposure as well as an altered circadian rhythm of body temperature. Chemogenetic activation of all Nts neurons in the brain induced a 4-5°C hypothermia, which could be blocked by Nts receptor antagonists in the preoptic area. Chemogenetic activation of preoptic neurotensinergic projections resulted in robust excitation of preoptic Adcyap1 neurons. Taken together, our data demonstrate that endogenously released Nts can induce potent hypothermia and that excitation of preoptic Adcyap1 neurons is the cellular mechanism that triggers this response.
Keywords: GABA; mouse; neuroscience; neurotensin; preoptic area; thermoregulation.
© 2024, Tabarean.
Conflict of interest statement
IT No competing interests declared
Figures





Update of
-
Opposing actions of co-released GABA and neurotensin on the activity of preoptic neurons and on body temperature.bioRxiv [Preprint]. 2024 Apr 17:2024.04.15.589556. doi: 10.1101/2024.04.15.589556. bioRxiv. 2024. Update in: Elife. 2024 Aug 29;13:RP98677. doi: 10.7554/eLife.98677. PMID: 38659782 Free PMC article. Updated. Preprint.
References
-
- Alexander MJ, Leeman SE. Widespread expression in adult rat forebrain of mRNA encoding high-affinity neurotensin receptor. The Journal of Comparative Neurology. 1998;402:475–500. - PubMed
-
- Frosini M, Valoti M, Sgaragli G. Changes in rectal temperature and ECoG spectral power of sensorimotor cortex elicited in conscious rabbits by i.c.v. injection of GABA, GABA(A) and GABA(B) agonists and antagonists. British Journal of Pharmacology. 2004;141:152–162. doi: 10.1038/sj.bjp.0705593. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

