Divergent downstream biosynthetic pathways are supported by <sc>L</sc>-cysteine synthases of Mycobacterium tuberculosis
- PMID: 39207917
- PMCID: PMC11361707
- DOI: 10.7554/eLife.91970
Divergent downstream biosynthetic pathways are supported by <sc>L</sc>-cysteine synthases of Mycobacterium tuberculosis
Abstract
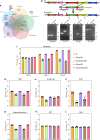
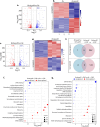
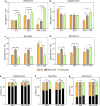
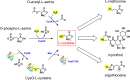
Mycobacterium tuberculosis's (Mtb) autarkic lifestyle within the host involves rewiring its transcriptional networks to combat host-induced stresses. With the help of RNA sequencing performed under various stress conditions, we identified that genes belonging to Mtb sulfur metabolism pathways are significantly upregulated during oxidative stress. Using an integrated approach of microbial genetics, transcriptomics, metabolomics, animal experiments, chemical inhibition, and rescue studies, we investigated the biological role of non-canonical L-cysteine synthases, CysM and CysK2. While transcriptome signatures of RvΔcysM and RvΔcysK2 appear similar under regular growth conditions, we observed unique transcriptional signatures when subjected to oxidative stress. We followed pool size and labelling (34S) of key downstream metabolites, viz. mycothiol and ergothioneine, to monitor L-cysteine biosynthesis and utilization. This revealed the significant role of distinct L-cysteine biosynthetic routes on redox stress and homeostasis. CysM and CysK2 independently facilitate Mtb survival by alleviating host-induced redox stress, suggesting they are not fully redundant during infection. With the help of genetic mutants and chemical inhibitors, we show that CysM and CysK2 serve as unique, attractive targets for adjunct therapy to combat mycobacterial infection.
Keywords: Mycobacterium; cysteine; infectious disease; microbiology; synthases; tuberculosis.
© 2023, Khan et al.
Conflict of interest statement
MK, DH, BS, YK, NS, DP, SD, DS, Ld, VN No competing interests declared
Figures










Update of
- doi: 10.1101/2023.10.03.560673
- doi: 10.7554/eLife.91970.1
- doi: 10.7554/eLife.91970.2
References
-
- Agren D, Schnell R, Oehlmann W, Singh M, Schneider G. Cysteine synthase (CysM) of Mycobacterium tuberculosis is an O-phosphoserine sulfhydrylase: evidence for an alternative cysteine biosynthesis pathway in mycobacteria. The Journal of Biological Chemistry. 2008;283:31567–31574. doi: 10.1074/jbc.M804877200. - DOI - PubMed
-
- Brunner K, Maric S, Reshma RS, Almqvist H, Seashore-Ludlow B, Gustavsson A-L, Poyraz Ö, Yogeeswari P, Lundbäck T, Vallin M, Sriram D, Schnell R, Schneider G. Inhibitors of the cysteine synthase CysM with antibacterial potency against dormant Mycobacterium tuberculosis. Journal of Medicinal Chemistry. 2016;59:6848–6859. doi: 10.1021/acs.jmedchem.6b00674. - DOI - PubMed
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- CRG/2018/001294/Science and Engineering Research Board
- JCB/2019/000015/Science and Engineering Research Board
- BT/PR13522/COE/34/27/2015/Department of Biotechnology, Ministry of Science and Technology, India
- FC001060/CRUK_/Cancer Research UK/United Kingdom
- FC001060/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

