Comparison of oral anticoagulants for stroke prevention in atrial fibrillation using the UK clinical practice research Datalink Aurum: A reference trial (ARISTOTLE) emulation study
- PMID: 39207948
- PMCID: PMC11361421
- DOI: 10.1371/journal.pmed.1004377
Comparison of oral anticoagulants for stroke prevention in atrial fibrillation using the UK clinical practice research Datalink Aurum: A reference trial (ARISTOTLE) emulation study
Abstract
Background: Stroke prevention guidance for patients with atrial fibrillation (AF) uses evidence generated from randomised controlled trials (RCTs). However, applicability to patient groups excluded from trials remains unknown. Real-world patient data provide an opportunity to evaluate outcomes in a trial analogous population of direct oral anticoagulants (DOACs) users and in patients otherwise excluded from RCTs; however, there remains uncertainty on the validity of methods and suitability of the data. Successful reference trial emulation can support the generation of evidence around treatment effects in groups excluded or underrepresented in trials. We used linked United Kingdom primary care data to investigate whether we could emulate the pivotal ARISTOTLE trial (apixaban versus warfarin) and extend the analysis to investigate the impact of warfarin time in therapeutic range (TTR) on results.
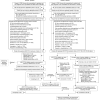
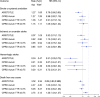
Methods and findings: Patients with AF in the UK Clinical Practice Research Datalink (CPRD Aurum) prescribed apixaban or warfarin from 1 January 2013 to 31 July 2019 were selected. ARISTOTLE eligibility criteria were applied to this population and matched to the RCT apixaban arm on baseline characteristics creating a trial-analogous apixaban cohort; this was propensity-score matched to warfarin users in the CPRD Aurum. ARISTOTLE outcomes were assessed using Cox proportional hazards regression stratified by prior warfarin exposure status during 2.5 years of patient follow-up and results benchmarked against the trial results before treatment effectiveness was further evaluated based on (warfarin) TTR. The dataset comprised 8,734 apixaban users and propensity-score matched 8,734 warfarin users. Results [hazard ratio (95% confidence interval)] confirmed apixaban noninferiority for stroke or systemic embolism (SE) [CPRD 0.98 (0.82,1.19) versus trial 0.79 (0.66,0.95)] and death from any cause [CPRD 1.03 (0.93,1.14) versus trial 0.89 (0.80,0.998)] but did not indicate apixaban superiority. Absolute event rates for stroke/SE were similar for apixaban in CPRD Aurum and ARISTOTLE (1.27%/year), whereas a lower event rate was observed for warfarin (CPRD Aurum 1.29%/year, ARISTOTLE 1.60%/year). Analysis by TTR suggested similar effectiveness of apixaban compared with poorly controlled warfarin (TTR < 0.75) for stroke/SE [0.91 (0.73, 1.14)], all-cause death [0.94 (0.84, 1.06)], and superiority for major bleeding [0.74 (0.63, 0.86)]. However, when compared with well-controlled warfarin (TTR ≥ 0.75), apixaban was associated with an increased hazard for all-cause death [1.20 (1.04, 1.37)], and there was no significant benefit for major bleeding [1.08 (0.90, 1.30)]. The main limitation of the study's methodology are the risk of residual confounding, channelling bias and attrition bias in the warfarin arm, and selection bias and misclassification in the analysis by TTR.
Conclusions: Analysis of noninterventional data generated results demonstrating noninferiority of apixaban versus warfarin consistent with prespecified benchmarking criteria. Unlike in ARISTOTLE, superiority of apixaban versus warfarin was not seen, possible due to the lower proportion of Asian patients and higher proportion of patients with well-controlled warfarin compared to ARISTOTLE. This methodological template can be used to investigate treatment effects of oral anticoagulants in patient groups excluded from or underrepresented in trials and provides a framework that can be adapted to investigate treatment effects for other conditions.
Copyright: © 2024 Powell et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
EMP was funded by a Medical Research Council studentship for this work, and is an employee of and holds stock in Compass Pathways outside the submitted work. UG is an employee of and holds stock in GSK. JT reports no conflict of interest and is supported by an unrestricted grant from GSK. LS reports grants from Wellcome, MRC, NIHR, BHF, Diabetes UK, ESRC, EU and GSK, personal fees from GSK and AstraZeneca, and is a trustee of the British Heart Foundation, outside the submitted work. PJB is supported by Barts Charity (MGU0504) and was supported by a GSK studentship at the time of writing. TBH reports no conflict of interest. AW reports no conflict of interest and is supported by a fellowship from British Heart Foundation (FS/19/19/34175). IJD reports grants, and holds stocks in GSK, outside the submitted work. KW has nothing to disclose.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

