Immunomodulatory potential of cytokine-licensed human bone marrow-derived mesenchymal stromal cells correlates with potency marker expression profile
- PMID: 39208292
- PMCID: PMC11630899
- DOI: 10.1093/stmcls/sxae053
Immunomodulatory potential of cytokine-licensed human bone marrow-derived mesenchymal stromal cells correlates with potency marker expression profile
Abstract
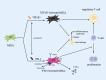
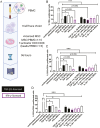
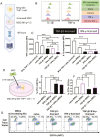
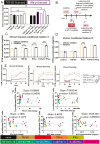
Cytokine(s) pre-activation/licensing is an effective way to enhance the immunomodulatory potency of mesenchymal stromal cells (MSCs). Currently, IFN-γ licensing received the most attention in comparison with other cytokines. After licensing human bone marrow-derived MSCs with pro-/anti-inflammatory cytokines IFN-γ, IL-1β, TNF-α, TGF-β1 alone or in combination, the in vitro immunomodulatory potency of these MSCs was studied by incubating with allogeneic T cells and macrophage-like THP-1 cells. In addition, immunomodulation-related molecules filtered by bioinformatics, complement 1 subcomponent (C1s), and interferon-induced GTP-binding protein Mx2 (MX2), were studied to verify whether to reflect the immunomodulatory potency. Herein, we reported that different cytokines cause different effects on the function of MSC. While TGF-β1 licensing enhances the capacity of MSCs to induce T cells with an immunosuppressive phenotype, IFN-γ-licensing strengthens the inhibitory effect of MSC on T cell proliferation. Both TGF-β1 and IFN-γ licensing can enhance the effect of MSC on reducing the expression of pro-inflammatory cytokines by M1 macrophage-like THP-1 cells. Interestingly, IFN-γ upregulates potential potency markers extracellular C1s and kynurenine (KYN) and intracellular MX2. These 3 molecules have the potential to reflect mesenchymal stromal cell immunomodulatory potency. In addition, we reported that there is a synergistic effect of TGF-β1 and IFN-γ in immunomodulation.
Keywords: IFN-γ; TGF-β1; immunomodulation; licensing; mesenchymal stromal cells; potency assay.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
The authors declared no potential conflicts of interest.
Figures



References
-
- Castro LL, Kitoko JZ, Xisto DG, et al.Multiple doses of adipose tissue-derived mesenchymal stromal cells induce immunosuppression in experimental asthma. Stem Cells Transl Med. 2020;9(2):250-260. https://doi.org/10.1002/sctm.19-0120 - DOI
-
- Sukho P, Hesselink JW, Kops N, et al.Human mesenchymal stromal cell sheets induce macrophages predominantly to an anti-inflammatory phenotype. Stem Cells Dev. 2018;27(13):922-934. https://doi.org/10.1089/scd.2017.0275 - DOI - PubMed
-
- Ge XH, Shi K, Hou J, et al.Galectin-1 secreted by bone marrow-derived mesenchymal stem cells mediates anti-inflammatory responses in acute airway disease. Exp Cell Res. 2021;407(1):112788. https://doi.org/10.1016/j.yexcr.2021.112788 - DOI - PubMed
-
- Ortiz-Virumbrales M, Menta R, Pérez LM, et al.Human adipose mesenchymal stem cells modulate myeloid cells toward an anti-inflammatory and reparative phenotype: role of IL-6 and PGE2. Stem Cell Res Therapy. 2020;11(1):462. https://doi.org/10.1186/s13287-020-01975-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

