The impact of different doses of oral iron supplementation during pregnancy: a pilot randomized trial
- PMID: 39208353
- PMCID: PMC11566866
- DOI: 10.1182/bloodadvances.2024013408
The impact of different doses of oral iron supplementation during pregnancy: a pilot randomized trial
Abstract
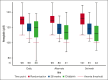
Oral iron is first-line medication for iron deficiency anemia in pregnancy. We conducted a pilot randomized trial to investigate the impact of different doses of oral iron supplementation started early in pregnancy on women without anemia for 4 main outcomes: recruitment and protocol compliance, adherence, maintenance of maternal hemoglobin, and side effects. At antenatal clinic visits, participants were allocated to 1 of 3 trial arms in a 1:1:1 ratio: 200 mg ferrous sulfate daily, alternate days, or 3 times per week. The participants were followed to delivery. Baseline characteristics of 300 recruited participants were well matched between trial arms. The mean proportion of tablets taken as expected per participant was 82.5% overall (72.3%, 89.6%, and 84.5% for the daily, alternate days, and 3 times a week arm, respectively). There was a lower overall adherence rate in the daily arm (47%) than in the alternate days (62%) and the 3 times per week (61%) arms. A reduction in hemoglobin between randomization and 28 weeks' gestation seemed smaller for the daily arm. A range of side effects were commonly reported at baseline before starting interventions and at later antenatal visits. Many side effects of iron overlapped with normal pregnancy symptoms. A daily iron dosing schedule might give the best opportunity for delivering an adequate iron load during pregnancy in women without anemia. Further randomized trials powered on clinical outcomes are needed to establish the clinical effectiveness of oral iron supplementation to prevent iron deficiency anemia. This study was registered (#ISRCTN12911644).
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the PANDA Collaborator Group appears in “Appendix.”
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical