A Targeted Methylation-Based Multicancer Early Detection Blood Test Preferentially Detects High-Grade Prostate Cancer While Minimizing Overdiagnosis of Indolent Disease
- PMID: 39208374
- PMCID: PMC11371104
- DOI: 10.1200/PO.24.00269
A Targeted Methylation-Based Multicancer Early Detection Blood Test Preferentially Detects High-Grade Prostate Cancer While Minimizing Overdiagnosis of Indolent Disease
Erratum in
-
Erratum: A Targeted Methylation-Based Multicancer Early Detection Blood Test Preferentially Detects High-Grade Prostate Cancer While Minimizing Overdiagnosis of Indolent Disease.JCO Precis Oncol. 2024 Oct;8:e2400637. doi: 10.1200/PO-24-00637. Epub 2024 Oct 16. JCO Precis Oncol. 2024. PMID: 39413340 Free PMC article. No abstract available.
Abstract
Purpose: Indolent prostate cancer (PCa) is prevalent in the intended use population (adults age 50-79 years) for blood-based multicancer early detection (MCED) tests. We examined the detectability of PCa by a clinically validated, targeted methylation-based MCED test.
Methods: Detectability by Gleason grade group (GG), clinical stage, association of detection status with tumor methylated fraction (TMeF), and overall survival (OS) were assessed in substudy 3 of Circulating Cell-Free Genome Atlas (CCGA; ClinicalTrials.gov identifier: NCT02889978) and PATHFINDER (ClinicalTrials.gov identifier: NCT04241796) studies.
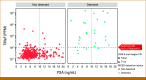
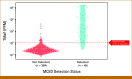
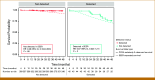
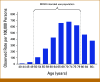
Results: Test sensitivity for PCa in substudy 3 of CCGA was 11.2% (47/420). The test detected 0 (0%) of 58 low-grade (GG1), 3 (1.9%) of 157 favorable intermediate-grade (GG2), 4 (5.1%) of 78 unfavorable intermediate-grade (GG3), and 36 (31.9%) of 113 high-grade (GG4 and 5) cancers and 3 (3.2%) of 95 stage I, 11 (4.7%) of 235 stage II, 7 (14.9%) of 47 stage III, and 22 (81.5%) of 27 stage IV cases. The median TMeF was higher for detected than nondetected cases (2,106.0 parts per million [PPM]; IQR, 349.8-24,376.3 v 24.4 PPM; IQR, 17.8-38.5; P < .05). Nondetected cases had better OS (P < .05; hazard ratio [HR], 0.263 [95% CI, 0.104 to 0.533]) and detected cases had similar survival (P = .2; HR, 0.672 [95% CI, 0.323 to 1.21]) compared with SEER adjusted for age, GG, and stage. Performance was similar in PATHFINDER, with no detected GG1/2 (0/13) or stage I/II (0/16) cases.
Conclusion: This MCED test preferentially detects high-grade, clinically significant PCa. Use in population-based screening programs in addition to standard-of-care screening is unlikely to exacerbate overdiagnosis of indolent PCa.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures

References
-
- Klein EA, Richards D, Cohn A, et al. : Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncol 32:1167-1177, 2021 - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

