Impact of a ketogenic diet on sleep quality in people with relapsing multiple sclerosis
- PMID: 39208520
- PMCID: PMC11393576
- DOI: 10.1016/j.sleep.2024.08.020
Impact of a ketogenic diet on sleep quality in people with relapsing multiple sclerosis
Abstract
Background: Sleep disturbance in MS is common and can significantly impair overall quality of life. The ketogenic diet (KD) associates with improved sleep quality in people living with epilepsy and may have similar benefits when used within MS; however, the impact of a KD on sleep in this population remains poorly defined.
Methods: Forty-five patients with relapsing MS enrolled into a 6-month KD intervention trial and completed self-reported assessments of sleep quality and sleep disorder symptoms prior to diet initiation and while on diet, using the Epworth Sleepiness Scale (ESS) and Sleep Disorders Symptom Checklist-25 (SDS). Participants who did not complete sleep assessments at baseline and 6-months were excluded from analysis. In addition to sleep metrics, data collection included anthropometrics and MS-related fatigue scores.
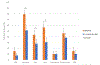
Results: Thirty-nine of 45 (87 %) participants completed the required sleep assessments. There was a mean reduction in ESS score of 1.90 (95 % CI [-2.85, -0.94], p < 0.001). Total SDS score decreased at 6-months on KD (-4.4, 95 % CI [-7.1, -1.7], p = 0.002), with improvements noted in insomnia (-1.55, 95 % CI [-2.66, -0.43], p = 0.008), obstructive sleep apnea (-0.91, 95 % CI [-1.57, -0.25], p = 0.008), and restless leg syndrome screening scores (-1.00, 95 % CI [-1.95, -0.051], p = 0.04). Sleep duration was unchanged on KD.
Conclusion: KD associates with improvements in daytime sleepiness, independent of sleep duration, and common comorbid sleep disorders in people living with relapsing MS. The findings herein support the benefits of KD on sleep quality and highlight the potential role of dietary therapeutics for sleep disorders in neurological disease.
Trial registration information: Registered on Clinicaltrials.gov under registration number NCT03718247, posted on Oct 24, 2018. First patient enrollment date: Nov 1, 2018. Link: https://clinicaltrials.gov/ct2/show/NCT03718247?term=NCT03718247&draw=2&rank=1.
Keywords: Apnea; Diet; Insomnia; Obesity; Restless leg; Sleep.
Copyright © 2024 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: D. Lehner-Gulotta is a consultant for Functional Formularies and Advanced Ketogenic Therapies. B. Banwell serves as a consultant to Novartis, Roche, UCB, Teva Neuroscience, Biogen, and Sanofi. AGC Bergqvist serves as a paid speaker for Nutricia North America. M.D. Goldman has served on the DSMB for Anokion SMC and Immunic. She has received consulting fees from Alexion, EMD Serono, Genetec, Greenwich Biosciences, Horizons, and Novartis. A.M. Morse has received research/grant support and consultancy fees from Flamel/Avadel, Takeda, Alkermes, Harmony Biosciences, LLC, Jazz Pharmaceuticals plc, NIH, UCB Pharmaceuticals and Geisinger Health Plan. She is a medical advisor for Neura Health. She is the CEO of DAMM Good Sleep, LLC. J.N. Brenton has served as a consultant to Cycle Pharmaceuticals and I-ACT for Children via support from Novartis. JNB's research is funded by the National Institute of Neurological Disorders and Stroke of the NIH (award number: K23NS116225) and by the UVA iTHRIV Scholars Program through the National Center for Advancing Translational Sciences of the NIH under award numbers UL1TR003015 and KL2TR003016.
Figures

References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

