Efficacy of BAFF inhibition and B-cell depletion in non-obese diabetic mice as a spontaneous model for Sjögren's disease
- PMID: 39209370
- PMCID: PMC11367362
- DOI: 10.1136/rmdopen-2024-004112
Efficacy of BAFF inhibition and B-cell depletion in non-obese diabetic mice as a spontaneous model for Sjögren's disease
Abstract
Introduction: The therapeutic interest of targeting B-cell activating factor (BAFF) in Sjögren's disease (SjD) can be suspected from the results of two phase II clinical trials but has not been evaluated in an animal model of the disease. We aimed to evaluate the therapeutic efficacy of this strategy on dryness and salivary gland (SG) infiltrates in the NOD mouse model of SjD.
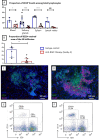
Material and methods: Female NOD mice between ages 10 and 18 weeks were treated with a BAFF-blocking monoclonal antibody, Sandy-2 or an isotype control. Dryness was measured by the stimulated salivary flow. Salivary lymphocytic infiltrates were assessed by immunohistochemistry. Blood, SGs, spleen and lymph-node lymphocyte subpopulations were analysed by flow cytometry. SG mRNA expression was analysed by transcriptomic analysis.
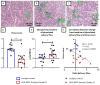
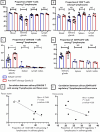
Results: BAFF inhibition significantly decreased SG lymphocytic infiltrates, which was inversely correlated with salivary flow. The treatment markedly decreased B-cell number in SGs, blood, lymph nodes and spleen and increased Foxp3+ regulatory and CD3+CD4-CD8- double negative T-cell numbers in SGs.
Conclusion: A monoclonal antibody blocking BAFF and depleting B cells had therapeutic effectiveness in the NOD mouse model of SjD. The increase in regulatory T-lymphocyte populations might underlie the efficacy of this treatment.
Keywords: B-Lymphocytes; Sjogren's Syndrome; T-Lymphocytes.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
