Inclusion, characteristics, and reporting of older adults in FDA registration studies of immunotherapy, 2018-2022
- PMID: 39209450
- PMCID: PMC11367347
- DOI: 10.1136/jitc-2024-009258
Inclusion, characteristics, and reporting of older adults in FDA registration studies of immunotherapy, 2018-2022
Abstract
Immune checkpoint inhibitors (ICI) have transformed the management of cancer, particularly for older adults, who constitute a majority of the global cancer patient population. This study aimed to assess the inclusion, characteristics, and reporting of older adults enrolled in Food and Drug Administration (FDA) registration clinical trials of ICI between 2018 and 2022. Clinical trials of ICI leading to an FDA approval in solid tumor oncology between 2018 and 2022 were included. Primary study reports and all available secondary publications were assessed. The availability and completeness of older subgroup data for protocol-defined clinical efficacy endpoints, health-related quality of life (HRQOL) and toxicity outcomes, and baseline characteristics were assessed according to predefined criteria which categorized reporting completeness hierarchically in relation to the availability of published data, including effect size, sample size, and measures of precision. 53 registration trials were included, involving a total of 37,094 participants. Most trials (64.2%) were of ICI combination therapy. 42.3% of patients were aged≥65 years; 11.1% were aged≥75. No trials specified an upper age limit for eligibility. 98.1% of trials excluded patients with European Cooperative Oncology Group performance status>1. 87.2% of primary efficacy endpoints and 17.9% of secondary efficacy endpoints were reported completely for older adults. Five studies (9.4%) reported baseline characteristics, three (6.1%) reported HRQOL assessments, and four (7.5%) reported toxicity outcomes completely among older subgroups. No trials conducted baseline geriatric assessments or reported geriatric-specific symptoms or quality of life scales. This analysis highlights significant deficits in the enrollment and reporting of older subgroups in pivotal trials of ICI therapy. The findings highlight an urgent need for improved reporting and inclusion standards in clinical trials of ICI to better inform treatment decisions for older adults.
Keywords: Geriatric; Immune Checkpoint Inhibitor; Immunotherapy.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: CME: None. RP: None. CS: None. MR: None. NMG-S: None. DW: None. NMLB: Advisory board: Pfizer, Abbott, Sanofi, Astellas; Travel grants: Exact Sciences, Pfizer, Lilly, Novartis; Speaker fees: Pfizer, AbbVie, Roche, Sanofi, Novartis, Servier, Gilead, AstraZeneca, Lilly.
Figures
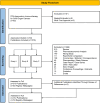
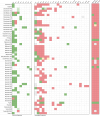
References
-
- International Agency for Research on Cancer Globocan 2020 global cancer observatory. [-Oct-2023]. http://gco.iarc.fr Available. Accessed.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
