Treatment algorithm for pulmonary arterial hypertension
- PMID: 39209476
- PMCID: PMC11525349
- DOI: 10.1183/13993003.01325-2024
Treatment algorithm for pulmonary arterial hypertension
Abstract
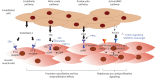
Pulmonary arterial hypertension leads to significant impairment in haemodynamics, right heart function, exercise capacity, quality of life and survival. Current therapies have mechanisms of action involving signalling via one of four pathways: endothelin-1, nitric oxide, prostacyclin and bone morphogenetic protein/activin signalling. Efficacy has generally been greater with therapeutic combinations and with parenteral therapy compared with monotherapy or nonparenteral therapies, and maximal medical therapy is now four-drug therapy. Lung transplantation remains an option for selected patients with an inadequate response to therapies.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: K.M. Chin reports grants and consultancy fees from Janssen, Merck, Gossamer Bio and United Therapeutics, support for attending meetings from Janssen, and is an associate editor for Circulation. S.P. Gaine reports grants from Janssen, Aerovent and Gossamer Bio, consultancy fees from Janssen, Merck, Gossamer Bio, United Therapeutics and Altavent, payment or honoraria for lectures, presentations, manuscript writing or educational events and support for attending meetings from Janssen, and participation on a data safety monitoring board or advisory board with Janssen. C. Gerges reports grants from OrphaCare, payment or honoraria for lectures, presentations, manuscript writing or educational events from AOPHealth, AstraZeneca, Janssen and Ferrer, and support for attending meetings from AOPHealth, AstraZeneca, Cordis, Janssen and MSD. Z-C. Jing has no potential conflicts of interest to disclose. S.C. Mathai reports consultancy fees from Janssen, United Therapeutics, Merck and Acceleron, participation on a data safety monitoring board or advisory board with Bayer, and a leadership role on the Patient Centered Outcomes Research Institute Rare Disease Advisory Panel. Y. Tamura reports grants from Nippon Shinyaku Co. Ltd and Mochida, consultancy fees from MSD, and payment or honoraria for lectures, presentations, manuscript writing or educational events from Nippon Shinyaku Co. Ltd and Janssen Pharmaceuticals. V.V. McLaughlin reports grants from Aerovate, Gossamer-Bio, Janssen, Keros, Merck and Sonovie, and consultancy fees from 35Pharma, Aerami, Aerovate, Caremark, L.L.C., Corvista, Gossamer Bio, Janssen, Keros, Merck, Riovant and United Therapeutics. O. Sitbon reports grants from Aerovate, AOP Orphan, Ferrer, Janssen and MSD, consultancy fees from Altavant/Enzyvant, AOP Orphan, Ferrer, Gossamer Bio, Janssen, Liquidia, MSD, Respira Therapeutics and Roivant Sciences, payment or honoraria for lectures, presentations, manuscript writing or educational events from Aerovate, AOP Orphan, Janssen, Ferrer and MSD, and participation on a data safety monitoring board or advisory board with Altavant/Enzyvant, Gossamer Bio, Janssen and Respira Therapeutics.
Figures

Comment in
-
The Seventh World Symposium on Pulmonary Hypertension: our journey to Barcelona.Eur Respir J. 2024 Oct 31;64(4):2401222. doi: 10.1183/13993003.01222-2024. Print 2024 Oct. Eur Respir J. 2024. PMID: 39209470 Free PMC article.
References
-
- Tremblay E, Gosselin C, Mai V, et al. Assessment of clinical worsening end points as a surrogate for mortality in pulmonary arterial hypertension: a systematic review and meta-analysis of randomized controlled trials. Circulation 2022; 146: 597–612. doi: 10.1161/CIRCULATIONAHA.121.058635 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
