Bithionol eliminates acute myeloid leukaemia stem-like cells by suppressing NF-κB signalling and inducing oxidative stress, leading to apoptosis and ferroptosis
- PMID: 39209810
- PMCID: PMC11362533
- DOI: 10.1038/s41420-024-02148-3
Bithionol eliminates acute myeloid leukaemia stem-like cells by suppressing NF-κB signalling and inducing oxidative stress, leading to apoptosis and ferroptosis
Abstract
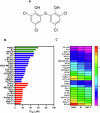
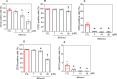
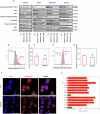
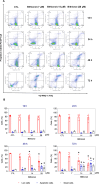
Acute myeloid leukaemia (AML) is a lethal bone marrow neoplasm caused by genetic alterations in blood cell progenitors. Leukaemic stem cells (LSCs) are responsible for the development of AML, drug resistance and relapse. Bithionol is an old anthelmintic drug with potential antibacterial, antiviral, antifungal, anti-Alzheimer, and antitumour properties. In this work, we focused on the anti-AML LSC properties of bithionol. This compound inhibited the viability of both solid and haematological cancer cells, suppressed AML stem-like cells, and inhibited AML growth in NSG mice at a dosage of 50 mg/kg, with tolerable systemic toxicity. Bithionol significantly reduced the levels of phospho-NF-κB p65 (Ser529) and phospho-NF-κB p65 (Ser536) and nuclear NF-κB p65 translocation in AML cells, indicating that this molecule can suppress NF-κB signalling. DNA fragmentation, nuclear condensation, cell shrinkage, phosphatidylserine externalisation, loss of transmembrane mitochondrial potential, caspase-3 activation and PARP-(Asp 214) cleavage were detected in bithionol-treated AML cells, indicating the induction of apoptosis. Furthermore, this compound increased mitochondrial superoxide levels, and bithionol-induced cell death was partially prevented by cotreatment with the selective ferroptosis inhibitor ferrostatin-1, indicating the induction of ferroptosis. In addition, bithionol synergised with venetoclax in AML cells, indicating the translational potential of bithionol to enhance the effects of venetoclax in patients with AML. Taken together, these data indicate that bithionol is a potential new anti-AML drug.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Cancer Stat Facts: Leukemia—Acute Myeloid Leukemia (AML). https://seer.cancer.gov/statfacts/html/amyl.html. Accessed 10 May 2024.
LinkOut - more resources
Full Text Sources
Research Materials

