XENTURION is a population-level multidimensional resource of xenografts and tumoroids from metastatic colorectal cancer patients
- PMID: 39209908
- PMCID: PMC11362617
- DOI: 10.1038/s41467-024-51909-2
XENTURION is a population-level multidimensional resource of xenografts and tumoroids from metastatic colorectal cancer patients
Abstract
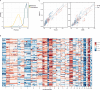
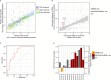
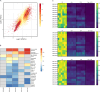
The breadth and depth at which cancer models are interrogated contribute to the successful clinical translation of drug discovery efforts. In colorectal cancer (CRC), model availability is limited by a dearth of large-scale collections of patient-derived xenografts (PDXs) and paired tumoroids from metastatic disease, where experimental therapies are typically tested. Here we introduce XENTURION, an open-science resource offering a platform of 128 PDX models from patients with metastatic CRC, along with matched PDX-derived tumoroids. Multidimensional omics analyses indicate that tumoroids retain extensive molecular fidelity with parental PDXs. A tumoroid-based trial with the anti-EGFR antibody cetuximab reveals variable sensitivities that are consistent with clinical response biomarkers, mirror tumor growth changes in matched PDXs, and recapitulate EGFR genetic deletion outcomes. Inhibition of adaptive signals upregulated by EGFR blockade increases the magnitude of cetuximab response. These findings illustrate the potential of large living biobanks, providing avenues for molecularly informed preclinical research in oncology.
© 2024. The Author(s).
Conflict of interest statement
L.T. has received research grants from Menarini, Merck KGaA, Merus, Pfizer, Servier, and Symphogen. The other authors declare no conflicts.
Figures




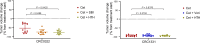
References
-
- Avolio, M. & Trusolino, L. Rational treatment of metastatic colorectal cancer: a reverse tale of men, mice, and culture dishes. Cancer Discov.11, 1644–1660 (2021). 10.1158/2159-8290.CD-20-1531 - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 22802/Associazione Italiana per la Ricerca sul Cancro (Italian Association for Cancer Research)
- 21091/Associazione Italiana per la Ricerca sul Cancro (Italian Association for Cancer Research)
- 22795/Associazione Italiana per la Ricerca sul Cancro (Italian Association for Cancer Research)
- 25040/Associazione Italiana per la Ricerca sul Cancro (Italian Association for Cancer Research)
- 23211/Associazione Italiana per la Ricerca sul Cancro (Italian Association for Cancer Research)
- 20697/Associazione Italiana per la Ricerca sul Cancro (Italian Association for Cancer Research)
- 21091/Associazione Italiana per la Ricerca sul Cancro (Italian Association for Cancer Research)
- 754923 COLOSSUS/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Societal Challenges | H2020 Health (H2020 Societal Challenges - Health, Demographic Change and Well-being)
- 731105 EDIReX/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Societal Challenges | H2020 Health (H2020 Societal Challenges - Health, Demographic Change and Well-being)
- 101058620 canSERV/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 724748 BEAT/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

