Rutin of Moringa oleifera as a potential inhibitor to Agaricus bisporus tyrosinase as revealed from the molecular dynamics of inhibition
- PMID: 39209920
- PMCID: PMC11362471
- DOI: 10.1038/s41598-024-69451-y
Rutin of Moringa oleifera as a potential inhibitor to Agaricus bisporus tyrosinase as revealed from the molecular dynamics of inhibition
Abstract
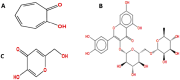
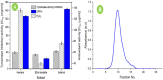
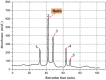
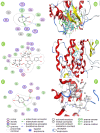
Tyrosinase is a binuclear copper-containing enzyme that catalyzes the conversation of monophenols to diphenols via o-hydroxylation and then the oxidation of o-diphenols to o-quinones which is profoundly linked to eukaryotic melanin synthesis and fruits browning. The hyperpigmentation due to unusual tyrosinase activity has gained growing health concern. Plants and their metabolites are considered promising and effective sources for potent antityrosinase enzymes. Hence, searching for potent, specific tyrosinase inhibitor from different plant extracts is an alternative approach in regulating overproduction of tyrosinase. Among the tested extracts, the hydro-alcoholic extract of Moringa oleifera L. leaves displayed the potent anti-tyrosinase activity (IC50 = 98.93 µg/ml) in a dose-dependent manner using L-DOPA as substrate; however, the kojic acid showed IC50 of 88.92 µg/ml. The tyrosinase-diphenolase (TYR-Di) kinetic analysis revealed mixed inhibition type for the Ocimum basilicum L. and Artemisia annua L. extracts, while the Coriandrum sativum L. extract displayed a non-competitive type of inhibition. Interestingly, the extract of Moringa oleifera L. leaves exhibited a competitive inhibition, low inhibition constant of free enzyme ( ) value and no Pan-Assay Interfering Substances, hinting the presence of strong potent inhibitors. The major putative antityrosinase compound in the extract was resolved, and chemically identified as rutin based on various spectroscopic analyses using UV-Vis, FTIR, mass spectrometry, and 1H NMR. The in silico computational molecular docking has been performed using rutin and A. bisporus tyrosinase (PDB code: 2Y9X). The binding energy of the predicted interaction between tropolone native ligand, kojic acid, and rutin against 2Y9X was respectively - 5.28, - 4.69, and - 7.75 kcal/mol. The docking simulation results revealed the reliable binding of rutin to the amino acid residues (ASN260, HIS259, SER282) in the tyrosinase catalytic site. Based on the developed results, rutin extracted from M. oleifera L. leaves has the capability to be powerful anti-pigment agent with a potential application in cosmeceutical area. In vivo studies are required to unravel the safety and efficiency of rutin as antityrosinase compound.
Keywords: Agaricus bisporus; Anti-pigment; Cosmetics; Flavonoid; Inhibition; Kinetics; Kojic acid; Rutin; Tyrosinase.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


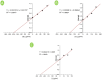
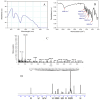
 ) and in the presence of different concentration of inhibitor, namely 50 µg/ml (
) and in the presence of different concentration of inhibitor, namely 50 µg/ml ( ), 100 µg/ml (
), 100 µg/ml ( ), and 150 µg/ml (
), and 150 µg/ml ( ).
).
References
-
- El-Shora, H. M. & El-Sharkawy, R. M. Evaluation of putative inducers and inhibitors toward tyrosinase from two Trichoderma species. Jordan J. Biol. Sci.13(1), 7–12 (2020).
-
- Laksmiani, N. P. L., Widiantara, I. W. A. & Pawarrangan, A. B. S. Potency of moringa (Moringa oleifera L.) leaves extract containing quercetin as a depigmentation agent inhibiting the tyrosinase enzyme using in silico and in vitro assay. Pharmacia69(1), 85–92 (2022). 10.3897/pharmacia.69.e73132 - DOI
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

