Ribosomal S6 kinase 1 regulates inflammaging via the senescence secretome
- PMID: 39210150
- PMCID: PMC11564105
- DOI: 10.1038/s43587-024-00695-z
Ribosomal S6 kinase 1 regulates inflammaging via the senescence secretome
Abstract
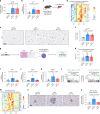
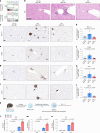
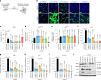
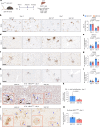
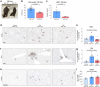
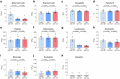
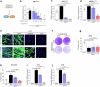
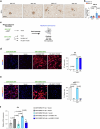
Inhibition of S6 kinase 1 (S6K1) extends lifespan and improves healthspan in mice, but the underlying mechanisms are unclear. Cellular senescence is a stable growth arrest accompanied by an inflammatory senescence-associated secretory phenotype (SASP). Cellular senescence and SASP-mediated chronic inflammation contribute to age-related pathology, but the specific role of S6K1 has not been determined. Here we show that S6K1 deletion does not reduce senescence but ameliorates inflammation in aged mouse livers. Using human and mouse models of senescence, we demonstrate that reduced inflammation is a liver-intrinsic effect associated with S6K deletion. Specifically, we show that S6K1 deletion results in reduced IRF3 activation; impaired production of cytokines, such as IL1β; and reduced immune infiltration. Using either liver-specific or myeloid-specific S6K knockout mice, we also demonstrate that reduced immune infiltration and clearance of senescent cells is a hepatocyte-intrinsic phenomenon. Overall, deletion of S6K reduces inflammation in the liver, suggesting that suppression of the inflammatory SASP by loss of S6K could underlie the beneficial effects of inhibiting this pathway on healthspan and lifespan.
© 2024. The Author(s).
Conflict of interest statement
Figures










References
MeSH terms
Substances
Grants and funding
- MC-A654-5QB40/RCUK | MRC | Medical Research Foundation
- 098565/Z/12/Z/Wellcome Trust (Wellcome)
- WT_/Wellcome Trust/United Kingdom
- MC_U120085810/RCUK | Medical Research Council (MRC)
- HepatoMetaboPath/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
LinkOut - more resources
Full Text Sources

