ENPP1 induces blood-brain barrier dysfunction and promotes brain metastasis formation in human epidermal growth factor receptor 2-positive breast cancer
- PMID: 39210244
- PMCID: PMC11726343
- DOI: 10.1093/neuonc/noae169
ENPP1 induces blood-brain barrier dysfunction and promotes brain metastasis formation in human epidermal growth factor receptor 2-positive breast cancer
Abstract
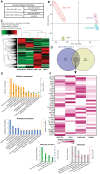
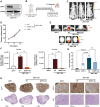
Background: Brain metastasis (BrM) is a devastating end-stage neurological complication that occurs in up to 50% of human epidermal growth factor receptor 2-positive (HER2+) breast cancer (BC) patients. Understanding how disseminating tumor cells manage to cross the blood-brain barrier (BBB) is essential for developing effective preventive strategies. We identified the ecto-nucleotidase ENPP1 (ectonucleotide pyrophosphatase/phosphodiesterase 1) as specifically enriched in the secretome of HER2+ brain metastatic cells, prompting us to explore its impact on BBB dysfunction and BrM formation.
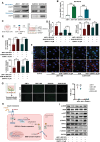
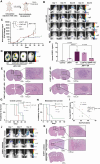
Methods: We used in vitro BBB and in vivo premetastatic mouse models to evaluate the effect of tumor-secreted ENPP1 on brain vascular permeability. BBB integrity was analyzed by real-time fluorescence imaging of 20 kDa Cy7.5-dextran extravasation and immunofluorescence staining of adherens and tight junction proteins. Pro-metastatic effects of ENPP1 were evaluated in an experimental brain metastatic model.
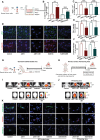
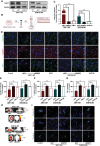
Results: Systemically secreted ENPP1 from primary breast tumors impaired the integrity of BBB with loss of tight and adherens junction proteins early before the onset of BrM. Mechanistically, ENPP1 induced endothelial cell dysfunction by impairing insulin signaling and its downstream AKT/GSK3β/β-catenin pathway. Genetic ablation of ENPP1 from HER2+ brain metastatic cells prevented endothelial cell dysfunction and reduced metastatic burden while prolonging the overall and metastasis-free survival of mice. Furthermore, plasmatic ENPP1 levels correlate with brain metastatic burden and inversely with overall survival.
Conclusions: We demonstrated that metastatic BC cells exploit the ENPP1 signaling for cell transmigration across the BBB and brain colonization. Our data implicate ENPP1 as a potential biomarker for poor prognosis and early detection of BrM in HER2+ BC.
Keywords: BBB dysfunction; ENPP1; HER2-positive breast cancer; brain metastasis; secretome.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Conflict of interest statement
None declared.
Figures

References
-
- Nayak L, Lee EQ, Wen PY.. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48–54. - PubMed
-
- Heitz F, Rochon J, Harter P, et al.Cerebral metastases in metastatic breast cancer: Disease-specific risk factors and survival. Ann Oncol. 2011;22(7):1571–1581. - PubMed
-
- Carvalho R, Paredes J, Ribeiro A.. Impact of breast cancer cells secretome on the brain metastatic niche remodeling. Semin Cell Dev Biol. 2020;60:294–301. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

