Polypharmacy and Guideline-Directed Medical Therapy Initiation Among Adults Hospitalized With Heart Failure
- PMID: 39210913
- PMCID: PMC11357976
- DOI: 10.1016/j.jacadv.2024.101126
Polypharmacy and Guideline-Directed Medical Therapy Initiation Among Adults Hospitalized With Heart Failure
Abstract
Background: Underprescribing of guideline-directed medical therapy (GDMT) for heart failure (HF) persists.
Objectives: The purpose of this study was to assess polypharmacy as a barrier to GDMT.
Methods: We examined participants hospitalized for HF with reduced ejection fraction and HF with mildly reduced ejection fraction between 2003 and 2017 from the Reasons for Geographic and Racial Differences in Stroke study. Participants were stratified by admission medication count-0 to 4, 5 to 9, and ≥10 medications. We examined GDMT use at admission, GDMT contraindications, and initiation of eligible indicated GDMT by medication count. We conducted a multivariable Poisson regression with robust standard errors to examine the association between medication count and GDMT initiation. GDMT included agents for HF with reduced ejection fraction/HF with mildly reduced ejection fraction, antiplatelet agents and statins for coronary artery disease, and anticoagulants for atrial fibrillation.
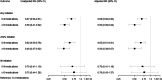
Results: Among 545 participants with HF, 34% were not taking a beta-blocker, 39% were not taking an angiotensin-converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor-neprilysin inhibitor, or hydralazine-isosorbide dinitrate, and 90% were not taking a mineralocorticoid receptor antagonist at admission; among participants with coronary artery disease, 36% were not taking an antiplatelet agent, and 38% were not taking a statin; and among participants with atrial fibrillation, 49% were not taking an anticoagulant. Polypharmacy was inversely associated with initiation of at least one indicated medication (5-9 medications: relative risk [RR]: 0.67; 95% CI: 0.56-0.82; P < 0.001; ≥10 medications: RR: 0.50; 95% CI: 0.39-0.64; P < 0.001) and initiation of at least half of indicated medications (5-9 medications: RR: 0.64; 95% CI: 0.51-0.81; P < 0.001; ≥10 medications: RR: 0.50; 95% CI: 0.38-0.67; P < 0.001).
Conclusions: Polypharmacy is an important barrier to GDMT.
Keywords: guideline-directed medical therapy; heart failure.
© 2024 The Authors.
Conflict of interest statement
This research project is supported by cooperative agreement U01 NS041588, co-funded by the 10.13039/100000065National Institute of Neurological Disorders and Stroke (NINDS) and the 10.13039/100000049National Institute on Aging (NIA), the 10.13039/100000002National Institutes of Health, and the 10.13039/100000016Department of Health and Human Services. Additional funding was provided by 10.13039/100000050National Heart, Lung, and Blood Institute (NHLBI) grant R01HL080477 (Dr Safford) and 10.13039/100000049NIA grant R03AG056446 (Dr Goyal). Representatives from the 10.13039/100000050NHLBI did not have any role in the design and conduct of the study, the collection, management, analysis, and interpretation of the data, or the preparation or approval of the manuscript. Dr Goyal is supported by 10.13039/100000968American Heart Association grant 20CDA35310455, 10.13039/100000049National Institute on Aging grant K76AG064428; has received personal fees for medicolegal consulting related to heart failure; has received consulting fees from Sensorum Health; and has received honoraria from Akcea Therapeutics, Inc. Dr Ambrosy is supported by a Mentored Patient-Oriented Research Career Development Award (K23HL150159) through the 10.13039/100000050National Heart, Lung, and Blood Institute and has received relevant research support through grants to his institution from 10.13039/100000046Abbott, 10.13039/100014389Amarin Pharma, 10.13039/100006520Edwards Lifesciences, 10.13039/501100022336Esperion, Lexicon, and 10.13039/100008272Novartis. Dr Levitan has received research funding from 10.13039/100002429Amgen, Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures



References
-
- Heidenreich P.A., Bozkurt B., Aguilar D., et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American heart association Joint committee on clinical practice guidelines. Circulation. 2022;145(18):E895–E1032. - PubMed
-
- Greene S.J., Butler J., Albert N.M., et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF registry. J Am Coll Cardiol. 2018;72(4):351–366. - PubMed
-
- Krantz M.J., Ambardekar A.V., Kaltenbach L., Hernandez A.F., Heidenreich P.A., Fonarow G.C. Patterns and Predictors of evidence-based medication Continuation among hospitalized heart failure patients (from Get with the guidelines–heart failure) Am J Cardiol. 2011;107(12):1818–1823. - PubMed
-
- Mebazaa A., Davison B., Chioncel O., et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomised, trial. Lancet. 2022;400(10367):1938–1952. - PubMed
-
- Bermingham M., Shanahan M.K., O’Connell E., et al. Aspirin use in heart failure: is low-dose therapy associated with mortality and morbidity benefits in a large community population? Circ Heart Fail. 2014;7(2):243–250. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

