Aryl hydrocarbon receptor: current perspectives on key signaling partners and immunoregulatory role in inflammatory diseases
- PMID: 39211042
- PMCID: PMC11358079
- DOI: 10.3389/fimmu.2024.1421346
Aryl hydrocarbon receptor: current perspectives on key signaling partners and immunoregulatory role in inflammatory diseases
Abstract
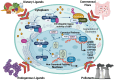
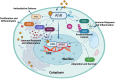
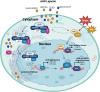
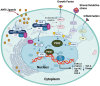
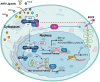
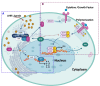
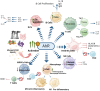
The aryl hydrocarbon receptor (AhR) is a versatile environmental sensor and transcription factor found throughout the body, responding to a wide range of small molecules originating from the environment, our diets, host microbiomes, and internal metabolic processes. Increasing evidence highlights AhR's role as a critical regulator of numerous biological functions, such as cellular differentiation, immune response, metabolism, and even tumor formation. Typically located in the cytoplasm, AhR moves to the nucleus upon activation by an agonist where it partners with either the aryl hydrocarbon receptor nuclear translocator (ARNT) or hypoxia-inducible factor 1β (HIF-1β). This complex then interacts with xenobiotic response elements (XREs) to control the expression of key genes. AhR is notably present in various crucial immune cells, and recent research underscores its significant impact on both innate and adaptive immunity. This review delves into the latest insights on AhR's structure, activating ligands, and its multifaceted roles. We explore the sophisticated molecular pathways through which AhR influences immune and lymphoid cells, emphasizing its emerging importance in managing inflammatory diseases. Furthermore, we discuss the exciting potential of developing targeted therapies that modulate AhR activity, opening new avenues for medical intervention in immune-related conditions.
Keywords: AhR; Aryl hydrocarbon receptor; immune regulation; inflammatory diseases; signaling pathways.
Copyright © 2024 Bahman, Choudhry, Al-Rashed, Al-Mulla, Sindhu and Ahmad.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
-
- Poland A, Glover E, Kende A. Stereospecific, high affinity binding of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem. (1976) 251:4936–46. doi: 10.1016/S0021-9258(17)33205-2 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

