Sacituzumab govitecan in triple-negative breast cancer: from bench to bedside, and back
- PMID: 39211043
- PMCID: PMC11357913
- DOI: 10.3389/fimmu.2024.1447280
Sacituzumab govitecan in triple-negative breast cancer: from bench to bedside, and back
Abstract
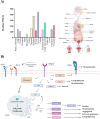
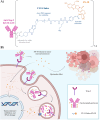
Triple-negative breast cancer (TNBC) represents a major therapeutic challenge due to its heterogeneous and aggressive phenotype, and limited target-specific treatment options. The trophoblast cell surface antigen (Trop-2), a transmembrane glycoprotein overexpressed in various cancers, has emerged as a promising target for TNBC. Sacituzumab govitecan (SG), an antibody-drug conjugate (ADC) that targets Trop-2, has recently entered treatment algorithms for advanced and metastatic TNBC, independently from Trop-2 expression status, with manageable toxicity. Despite the impressive results, questions remain unsolved regarding its efficacy, safety profile, and Trop-2 biological role in cancer. Currently, Trop-2 cannot be designated as a predictive biomarker in SG treatment, albeit its expression correlates with disease outcome, yet its levels are not uniform across all TNBCs. Additionally, data regarding Trop-2 expression variations in primary and metastatic sites, and its interplay with other biomarkers are still ambiguous but mandatory in light of future applications of SG in other indications and settings. This poses the questions of a careful evaluation of the efficacy and toxicity profile of SG in such early stages of disease, and in personalized and combinatorial strategies. Research and clinical data are mandatory to address SG drawbacks and minimize its benefits, to realize its full potential as therapeutic agent in different epithelial tumors.
Keywords: Trop-2; antibody-drug conjugate (ADC); immunotherapy; metastatic TNBC; sacituzumab govitecan (IMMU-132); target therapeutics; triple negative breast cancer (TNBC).
Copyright © 2024 Rossi, Turati, Rosato and Carpanese.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

