Stem-like CD8+ T cells in cancer
- PMID: 39211052
- PMCID: PMC11357971
- DOI: 10.3389/fimmu.2024.1426418
Stem-like CD8+ T cells in cancer
Abstract
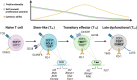
Stem-like CD8+ T cells (TSL) are a subset of immune cells with superior persistence and antitumor immunity. They are TCF1+ PD-1+ and important for the expansion of tumor specific CD8+ T cells in response to checkpoint blockade immunotherapy. In acute infections, naïve CD8+ T cells differentiate into effector and memory CD8+ T cells; in cancer and chronic infections, persistent antigen stimulation can lead to T cell exhaustion. Recent studies have highlighted the dichotomy between late dysfunctional (or exhausted) T cells (TLD) that are TCF1- PD-1+ and self-renewing TCF1+ PD-1+ TSL from which they derive. TCF1+ TSL cells are considered to have stem cell-like properties akin to memory T cell populations and can give rise to cytotoxic effector and transitory T cell phenotypes (TTE) which mediate tumor control. In this review, we will discuss recent advances made in research on the formation and expansion of TSL, as well as distinct niches required for their differentiation and maintenance in the setting of cancer. We will also discuss potential strategies to generate these cells, with clinical implications for stemness enhancement in vaccine design, immune checkpoint blockade (ICB), and adoptive T cell therapies.
Keywords: cancer models; chronic viral infection; immune; stem-like CD8 T cells (TSL); tertiary lymphoid structure (TLS); tumor microenvironment (TME).
Copyright © 2024 Steiner, Denlinger, Huang and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Siddiqui I, Schaeuble K, Chennupati V, Fuertes Marraco SA, Calderon-Copete S, Pais Ferreira D, et al. . Intratumoral Tcf1(+)PD-1(+)CD8(+) T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity. (2019) 50:195–211 e110. doi: 10.1016/j.immuni.2018.12.021 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

