Targeting and activation of macrophages in leishmaniasis. A focus on iron oxide nanoparticles
- PMID: 39211053
- PMCID: PMC11357945
- DOI: 10.3389/fimmu.2024.1437430
Targeting and activation of macrophages in leishmaniasis. A focus on iron oxide nanoparticles
Abstract
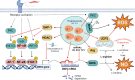
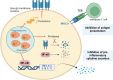
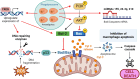
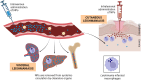
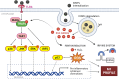
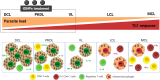
Macrophages play a pivotal role as host cells for Leishmania parasites, displaying a notable functional adaptability ranging from the proinflammatory, leishmanicidal M1 phenotype to the anti-inflammatory, parasite-permissive M2 phenotype. While macrophages can potentially eradicate amastigotes through appropriate activation, Leishmania employs diverse strategies to thwart this activation and redirect macrophages toward an M2 phenotype, facilitating its survival and replication. Additionally, a competition for iron between the two entities exits, as iron is vital for both and is also implicated in macrophage defensive oxidative mechanisms and modulation of their phenotype. This review explores the intricate interplay between macrophages, Leishmania, and iron. We focus the attention on the potential of iron oxide nanoparticles (IONPs) as a sort of immunotherapy to treat some leishmaniasis forms by reprogramming Leishmania-permissive M2 macrophages into antimicrobial M1 macrophages. Through the specific targeting of iron in macrophages, the use of IONPs emerges as a promising strategy to finely tune the parasite-host interaction, endowing macrophages with an augmented antimicrobial arsenal capable of efficiently eliminating these intrusive microbes.
Keywords: host-directed therapies; iron oxide nanoparticles; leishmania; macrophages; target; targeted delivery.
Copyright © 2024 Palomino-Cano, Moreno, Irache and Espuelas.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Natural phytochemicals reverting M2 to M1 macrophages: A novel alternative leishmaniasis therapy.Microb Pathog. 2025 Mar;200:107311. doi: 10.1016/j.micpath.2025.107311. Epub 2025 Jan 23. Microb Pathog. 2025. PMID: 39863089 Review.
-
New Therapeutic Tools to Shape Monocyte Functional Phenotypes in Leishmaniasis.Front Immunol. 2021 Jun 25;12:704429. doi: 10.3389/fimmu.2021.704429. eCollection 2021. Front Immunol. 2021. PMID: 34249011 Free PMC article. Review.
-
Leishmania and its quest for iron: An update and overview.Mol Biochem Parasitol. 2017 Jan;211:15-25. doi: 10.1016/j.molbiopara.2016.12.004. Epub 2016 Dec 15. Mol Biochem Parasitol. 2017. PMID: 27988301 Review.
-
Macrophage Polarization in the Skin Lesion Caused by Neotropical Species of Leishmania sp.J Immunol Res. 2021 Apr 10;2021:5596876. doi: 10.1155/2021/5596876. eCollection 2021. J Immunol Res. 2021. PMID: 33937417 Free PMC article.
-
Chemokines in host-parasite interactions in leishmaniasis.Trends Parasitol. 2006 Jan;22(1):32-40. doi: 10.1016/j.pt.2005.11.010. Epub 2005 Nov 23. Trends Parasitol. 2006. PMID: 16310413 Review.
Cited by
-
Oxidative Stress and Survival of Leishmania spp.: A Relationship of Inverse Proportionality for Disease Outcome.Expert Rev Mol Med. 2025 Jun 20;27:e21. doi: 10.1017/erm.2025.10010. Expert Rev Mol Med. 2025. PMID: 40538050 Free PMC article. Review.
References
-
- World Health Organization . Ending the neglect to attain the sustainable development goals: a road map for neglected tropical diseases 2021–2030 (2020). Available online at: https://www.who.int/publications/i/item/9789240010352 (Accessed May 15, 2024).
-
- Ruiz-Postigo JA, Jain S, Madjou S, Virrey Agua JF, Maia-Elkhoury AN, Valadas S, et al. . Global leishmaniasis surveillance, 2022: assessing trends over the past 10 years (2023). Available online at: https://www.who.int/publications/i/item/who-wer9840-471-487 (Accessed May 15, 2024).
-
- Kamran M, Bhattacharjee R, Das S, Mukherjee S, Ali N. The paradigm of intracellular parasite survival and drug resistance in leishmanial parasite through genome plasticity and epigenetics: Perception and future perspective. Front Cell Infect Microbiol. (2023) 13:1001973. doi: 10.3389/fcimb.2023.1001973 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

