Establishment of a novel amyotrophic lateral sclerosis patient (TARDBP N345K/+)-derived brain microvascular endothelial cell model reveals defective Wnt/β-catenin signaling: investigating diffusion barrier dysfunction and immune cell interaction
- PMID: 39211392
- PMCID: PMC11357944
- DOI: 10.3389/fcell.2024.1357204
Establishment of a novel amyotrophic lateral sclerosis patient (TARDBP N345K/+)-derived brain microvascular endothelial cell model reveals defective Wnt/β-catenin signaling: investigating diffusion barrier dysfunction and immune cell interaction
Abstract
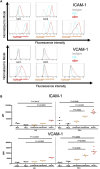
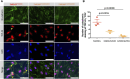
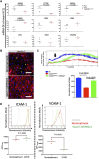
Amyotrophic lateral sclerosis (ALS) is a major neurodegenerative disease for which there is currently no curative treatment. The blood-brain barrier (BBB), multiple physiological functions formed by mainly specialized brain microvascular endothelial cells (BMECs), serves as a gatekeeper to protect the central nervous system (CNS) from harmful molecules in the blood and aberrant immune cell infiltration. The accumulation of evidence indicating that alterations in the peripheral milieu can contribute to neurodegeneration within the CNS suggests that the BBB may be a previously overlooked factor in the pathogenesis of ALS. Animal models suggest BBB breakdown may precede neurodegeneration and link BBB alteration to the disease progression or even onset. However, the lack of a useful patient-derived model hampers understanding the pathomechanisms of BBB dysfunction and the development of BBB-targeted therapies. In this study, we differentiated BMEC-like cells from human induced pluripotent stem cells (hiPSCs) derived from ALS patients to investigate BMEC functions in ALS patients. TARDBP N345K/+ carrying patient-derived BMEC-like cells exhibited increased permeability to small molecules due to loss of tight junction in the absence of neurodegeneration or neuroinflammation, highlighting that BMEC abnormalities in ALS are not merely secondary consequences of disease progression. Furthermore, they exhibited increased expression of cell surface adhesion molecules like ICAM-1 and VCAM-1, leading to enhanced immune cell adhesion. BMEC-like cells derived from hiPSCs with other types of TARDBP gene mutations (TARDBP K263E/K263E and TARDBP G295S/G295S) introduced by genome editing technology did not show such BMEC dysfunction compared to the isogenic control. Interestingly, transactive response DNA-binding protein 43 (TDP-43) was mislocalized to cytoplasm in TARDBP N345K/+ carrying model. Wnt/β-catenin signaling was downregulated in the ALS patient (TARDBP N345K/+)-derived BMEC-like cells and its activation rescued the leaky barrier phenotype and settled down VCAM-1 expressions. These results indicate that TARDBP N345K/+ carrying model recapitulated BMEC abnormalities reported in brain samples of ALS patients. This novel patient-derived BMEC-like cell is useful for the further analysis of the involvement of vascular barrier dysfunctions in the pathogenesis of ALS and for promoting therapeutic drug discovery targeting BMEC.
Keywords: TDP-43; Wnt/β-catenin signaling; amyotrophic lateral sclerosis; blood-brain barrier; human induced pluripotent stem cells.
Copyright © 2024 Matsuo, Nagamatsu, Nagata, Umeda, Shiota, Morimoto, Suzuki, Aoki, Okano, Nakamori and Nishihara.
Conflict of interest statement
HO reports grants and personal fees from K Pharma, Inc. during the conduct of the study; and personal fees from Sanbio Co. Ltd., outside the submitted work; In addition, HO has a patent on a therapeutic agent for amyotrophic lateral sclerosis and composition for treatment licensed to K Pharma, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Cheemala A., Kimble A. L., Tyburski J. D., Leclair N. K., Zuberi A. R., Murphy M., et al. (2023). Loss of endothelial TDP-43 leads to blood brain barrier defects in mouse models of amyotrophic lateral sclerosis and frontotemporal dementia, bioRxiv, 2023.2012.2013.571184. 10.1101/2023.12.13.571184 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

