Profiling biomanufactured extracellular vesicles of human forebrain spheroids in a Vertical-Wheel Bioreactor
- PMID: 39211409
- PMCID: PMC11350274
- DOI: 10.1002/jex2.70002
Profiling biomanufactured extracellular vesicles of human forebrain spheroids in a Vertical-Wheel Bioreactor
Abstract
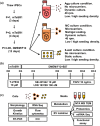
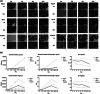
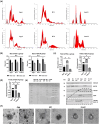
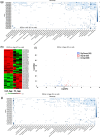
Extracellular vesicles (EVs) secreted by human brain cells have great potential as cell-free therapies in various diseases, including stroke. However, because of the significant amount of EVs needed in preclinical and clinical trials, EV application is still challenging. Vertical-Wheel Bioreactors (VWBRs) have designed features that allow for scaling up the generation of human forebrain spheroid EVs under low shear stress. In this study, EV secretion by human forebrain spheroids derived from induced pluripotent stem cells as 3D aggregates and on Synthemax II microcarriers in VWBRs were investigated with static aggregate culture as a control. The spheroids were characterized by metabolite and transcriptome analysis. The isolated EVs were characterized by nanoparticle tracking analysis, electron microscopy, and Western blot. The EV cargo was analyzed using proteomics and miRNA sequencing. The in vitro functional assays of an oxygen and glucose-deprived stroke model were conducted. Proof of concept in vivo study was performed, too. Human forebrain spheroid differentiated on microcarriers showed a higher growth rate than 3D aggregates. Microcarrier culture had lower glucose consumption per million cells and lower glycolysis gene expression but higher EV biogenesis genes. EVs from the three culture conditions showed no differences in size, but the yields from high to low were microcarrier cultures, dynamic aggregates, and static aggregates. The cargo is enriched with proteins (proteomics) and miRNAs (miRNA-seq), promoting axon guidance, reducing apoptosis, scavenging reactive oxygen species, and regulating immune responses. Human forebrain spheroid EVs demonstrated the ability to improve recovery in an in vitro stroke model and in vivo. Human forebrain spheroid differentiation in VWBR significantly increased the EV yields (up to 240-750 fold) and EV biogenesis compared to static differentiation due to the dynamic microenvironment and metabolism change. The biomanufactured EVs from VWBRs have exosomal characteristics and more therapeutic cargo and are functional in in vitro assays, which paves the way for future in vivo stroke studies.
Keywords: Vertical‐Wheel Bioreactor; aggregates; extracellular vesicles; human forebrain spheroids; microcarriers; multi‐omics.
© 2024 The Author(s). Journal of Extracellular Biology published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Abdollahi, S. (2021). Extracellular vesicles from organoids and 3D culture systems. Biotechnology and Bioengineering, 118(3), 1029–1049. - PubMed
-
- Apodaca, L. A. , Baddour, A. A. D. , Garcia, C. , Alikhani, L. , Giedzinski, E. , Ru, N. , Agrawal, A. , Acharya, M. M. , & Baulch, J. E. (2021). Human neural stem cell‐derived extracellular vesicles mitigate hallmarks of Alzheimer's disease. Alzheimer's Research & Therapy, 13(1), 1–18. - PMC - PubMed
-
- Arthur, P. , Kandoi, K. , Sun, L. , Kalvala, A. , Kuthleria, S. , Bhattacharaya, S. , Kulkarni, T. , Nimma, R. , Li, Y. , Lamba, D. A. , & Sing, M. (2023). Biophysical, molecular and proteomic profiling of human retinal organoids derived exosomes. Pharmacological Research, 40(4), 801–816. - PMC - PubMed
-
- Borys, B. S. , Dang, T. , So, T. , Rohani, L. , Revay, T. , Walsh, T. , Thompson, M. , Argiropoulos, B. , Rancourt, D. E. , & Jung, S. (2021). Overcoming bioprocess bottlenecks in the large‐scale expansion of high‐quality hiPSC aggregates in vertical‐wheel stirred suspension bioreactors. Stem Cell Research & Therapy, 12(1), 1–19. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
