Safety and efficacy of phosphodiesterase-5 (PDE-5) inhibitors in fetal growth restriction: a systematic literature review and meta-analysis
- PMID: 39211421
- PMCID: PMC11357966
- DOI: 10.3389/jpps.2024.13206
Safety and efficacy of phosphodiesterase-5 (PDE-5) inhibitors in fetal growth restriction: a systematic literature review and meta-analysis
Abstract
Introduction: Fetal growth restriction (FGR) is associated with a higher risk of perinatal morbidity and mortality, as well as long-term health issues in newborns. Currently, there is no effective medicine for FGR. Phosphodiesterase-5 (PDE-5) inhibitors have been shown in pre-clinical studies to improve FGR. This study aimed to evaluate the latest evidence about the clinical outcomes and safety of PDE-5 inhibitors for the management of FGR. Methods: Eight databases (PubMed, Embase, Medline, Web of Science, Cochrane Library, Chinese National Knowledge Infrastructure, Chinese Biomedical Database and WangFang Database) were searched for English and Chinese articles published from the database inception to December 2023. Randomized controlled trials (RCTs) reporting the use of PDE-5 inhibitors in FGR were included. The quality of the RCTs was assessed using the Cochrane Risk of Bias Tool. Odds ratio and mean difference (MD) (95% confidence intervals) were pooled for meta-analysis. Results: From 253 retrieved publications, 16 studies involving 1,492 pregnant women met the inclusion criteria. Only sildenafil (15 RCTs) and tadalafil (1 RCT) were studied for FGR. Compared with the control group (placebo, no treatment, or other medication therapies), sildenafil increased birth weight, pregnancy prolongation and umbilical artery pulsatility indices. However, it also increased the risk of pulmonary hypertension in newborns, as well as headache and flushing/rash in mothers. There were no significant differences in gestation age, perinatal mortality or major neonatal morbidity, stillbirth, neonate death, infants admitted to neonatal intensive care unit, intraventricular hemorrhage and necrotizing enterocolitis in infants, as well as pregnancy hypertension and gastrointestinal side effects in mothers between the treatment and the control groups. Discussion: Sildenafil was the most investigated PDE-5 inhibitors for FGR. Current evidence suggests that sildenafil can improve birth weight and duration of pregnancy but at the same time increase the risk of neonatal pulmonary hypertension. It remains uncertain whether the benefits of sildenafil in FGR outweigh the risks and further high-quality RCTs are warranted. Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=325909.
Keywords: fetal growth restriction; intrauterine growth restriction; phosphodiesterase-5 inhibitors; sildenafil; tadalafil.
Copyright © 2024 Liu, Un, Bai, Chan, Zeng, Lei, Li and Ung.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
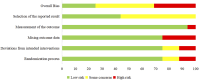
Figures
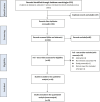


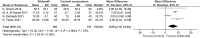




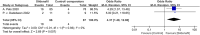
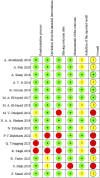
References
-
- Xia YH, Hu YL, Bo QH, Ming SL, Ming ZM. Expert consensus on fetal growth restriction (2019 edition). Chin J Prenatal Diagn (Electronic Version) (2019) 11(04):78–98. 10.3760/cma.j.issn.1007-9408.2019.06.001 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

