Dual effect of N-terminal deletion of cardiac myosin essential light chain in mitigating cardiomyopathy
- PMID: 39211545
- PMCID: PMC11357882
- DOI: 10.1016/j.isci.2024.110591
Dual effect of N-terminal deletion of cardiac myosin essential light chain in mitigating cardiomyopathy
Abstract
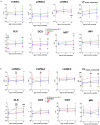
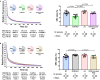
We investigated the role of the N-terminus (residues 1-43) of the myosin essential light chain (N-ELC) in regulating cardiac function in hypertrophic (HCM-A57G) and restrictive (RCM-E143K) cardiomyopathy mice. Both models were cross-genotyped with N-ELC-truncated Δ43 mice, and the offspring were studied using echocardiography and muscle contractile mechanics. In A57G×Δ43 mice, Δ43 expression improved heart function and reduced hypertrophy and fibrosis. No improvements were seen in E143K×Δ43 compared to RCM-E143K mice. HCM-mutant pathology involved an impaired N-ELC tension sensor, disrupted N-ELC-actin interactions, an altered force-pCa relationship, and a destabilized myosin's super-relaxed state. Removal of the malfunctioning N-ELC sensor led to functional rescue in HCM-truncated mutant hearts. However, the RCM mutation could not be rescued by N-ELC deletion, likely due to its proximity to the myosin motor domain, affecting lever-arm rigidity and myosin function. This study provides insights into the role of N-ELC in the development and potential rescue of ELC-mutant cardiomyopathy.
Keywords: Biochemical mechanism; Medical biochemistry; Molecular medicine; Pathophysiology.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Yadav S., Sitbon Y.H., Kazmierczak K., Szczesna-Cordary D. Hereditary heart disease: pathophysiology, clinical presentation, and animal models of HCM, RCM, and DCM associated with mutations in cardiac myosin light chains. Pflügers Archiv. 2019;471:683–699. doi: 10.1007/s00424-019-02257-4. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

