An Enzymatic Oxidation Cascade Converts δ-Thiolactone Anthracene to Anthraquinone in the Biosynthesis of Anthraquinone-Fused Enediynes
- PMID: 39211597
- PMCID: PMC11350584
- DOI: 10.1021/jacsau.4c00279
An Enzymatic Oxidation Cascade Converts δ-Thiolactone Anthracene to Anthraquinone in the Biosynthesis of Anthraquinone-Fused Enediynes
Abstract
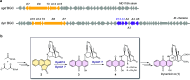
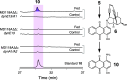
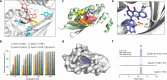
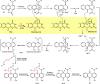
Anthraquinone-fused enediynes are anticancer natural products featuring a DNA-intercalating anthraquinone moiety. Despite recent insights into anthraquinone-fused enediyne (AQE) biosynthesis, the enzymatic steps involved in anthraquinone biogenesis remain to be elucidated. Through a combination of in vitro and in vivo studies, we demonstrated that a two-enzyme system, composed of a flavin adenine dinucleotide (FAD)-dependent monooxygenase (DynE13) and a cofactor-free enzyme (DynA1), catalyzes the final steps of anthraquinone formation by converting δ-thiolactone anthracene to hydroxyanthraquinone. We showed that the three oxygen atoms in the hydroxyanthraquinone originate from molecular oxygen (O2), with the sulfur atom eliminated as H2S. We further identified the key catalytic residues of DynE13 and A1 by structural and site-directed mutagenesis studies. Our data support a catalytic mechanism wherein DynE13 installs two oxygen atoms with concurrent desulfurization and decarboxylation, whereas DynA1 acts as a cofactor-free monooxygenase, installing the final oxygen atom in the hydroxyanthraquinone. These findings establish the indispensable roles of DynE13 and DynA1 in AQE biosynthesis and unveil novel enzymatic strategies for anthraquinone formation.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Ando T.; Ishii M.; Kajiura T.; Kameyama T.; Miwa K.; Sugiura Y. A new non-protein enediyne antibiotic N1999A2: Unique enediyne chromophore similar to neocarzinostatin and DNA cleavage feature. Tetrahedron Lett. 1998, 39 (36), 6495–6498. 10.1016/S0040-4039(98)01383-5. - DOI
LinkOut - more resources
Full Text Sources
