Precision Nanovaccines for Potent Vaccination
- PMID: 39211600
- PMCID: PMC11350730
- DOI: 10.1021/jacsau.4c00568
Precision Nanovaccines for Potent Vaccination
Abstract
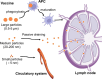
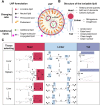
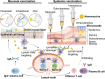
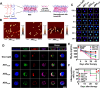
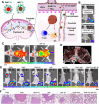
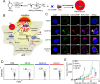
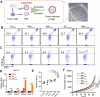
Compared with traditional vaccines, nanoparticulate vaccines are especially suitable for delivering antigens of proteins, peptides, and nucleic acids and facilitating lymph node targeting. Moreover, apart from improving pharmacokinetics and safety, nanoparticulate vaccines assist antigens and molecular adjuvants in crossing biological barriers, targeting immune organs and antigen-presenting cells (APC), controlled release, and cross-presentation. However, the process that stimulates and orchestrates the immune response is complicated, involving spatiotemporal interactions of multiple cell types, including APCs, B cells, T cells, and macrophages. The performance of nanoparticulate vaccines also depends on the microenvironments of the target organs or tissues in different populations. Therefore, it is necessary to develop precise nanoparticulate vaccines that accurately regulate vaccine immune response beyond simply improving pharmacokinetics. This Perspective summarizes and highlights the role of nanoparticulate vaccines with precise size, shape, surface charge, and spatial management of antigen or adjuvant for a precision vaccination in regulating the distribution, targeting, and immune response. It also discusses the importance of the rational design of nanoparticulate vaccines based on the anatomical and immunological microstructure of the target tissues. Moreover, the target delivery and controlled release of nanovaccines should be taken into consideration in designing vaccines for achieving precise immune responses. Additionally, it shows that the nanovaccines remodel the suppressed tumor environment and modulate various immune cell responses which are also essential.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
