Recent advances in ionic thermoelectric systems and theoretical modelling
- PMID: 39211742
- PMCID: PMC11348834
- DOI: 10.1039/d4sc04158e
Recent advances in ionic thermoelectric systems and theoretical modelling
Abstract
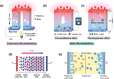
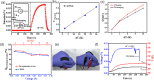
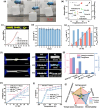
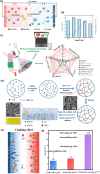
Converting waste heat from solar radiation and industrial processes into useable electricity remains a challenge due to limitations of traditional thermoelectrics. Ionic thermoelectric (i-TE) materials offer a compelling alternative to traditional thermoelectrics due to their excellent ionic thermopower, low thermal conductivity, and abundant material options. This review categorizes i-TE materials into thermally diffusive and thermogalvanic types, with an emphasis on the former due to its superior thermopower. This review also highlights the i-TE materials for creating ionic thermoelectric supercapacitors (ITESCs) that can generate significantly higher voltages from low-grade heat sources compared to conventional technologies. Additionally, it explores thermogalvanic cells and combined devices, discussing key optimization parameters and theoretical modeling approaches for maximizing material and device performance. Future directions aim to enhance i-TE material performance and address low energy density challenges for flexible and wearable applications. Herein, the cutting-edge of i-TE materials are comprehensively outlined, empowering researchers to develop next-generation waste heat harvesting technologies for a more sustainable future.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures













References
-
- Sun S. Li M. Shi X. L. Chen Z. G. Advances in ionic thermoelectrics: from materials to devices. Adv. Energy Mater. 2023;13(9):2203692. doi: 10.1002/aenm.202203692. - DOI
-
- Zhou Z. Wan Y. Zi J. Ye G. Jin T. Geng X. Zhuang W. Yang P. Flexible hydrogel with a coupling enhanced thermoelectric effect for low-grade heat harvest. Mater. Today Sustain. 2023;21:100293.
-
- Gao C. Yin Y. Zheng L. Liu Y. Sim S. He Y. Zhu C. Liu Z. Lee H. W. Yuan Q. Engineering the electrochemical temperature coefficient for efficient low-grade heat harvesting. Adv. Funct. Mater. 2018;28(35):1803129. doi: 10.1002/adfm.201803129. - DOI
-
- Muddasar M. Mushtaq M. Beaucamp A. Kennedy T. Culebras M. Collins M. N. Synthesis of sustainable lignin precursors for hierarchical porous carbons and their efficient performance in energy storage applications. ACS Sustain. Chem. Eng. 2024;12:2352–2363. doi: 10.1021/acssuschemeng.3c07202. - DOI - PMC - PubMed
-
- Wei J. Yang L. Ma Z. Song P. Zhang M. Ma J. Yang F. Wang X. Review of current high-ZT thermoelectric materials. J. Mater. Sci. 2020;55:12642–12704. doi: 10.1007/s10853-020-04949-0. - DOI
Publication types
LinkOut - more resources
Full Text Sources

