Enhanced enrichment of extracellular vesicles for laboratory and clinical research from drop-sized blood samples
- PMID: 39211743
- PMCID: PMC11358096
- DOI: 10.3389/fmolb.2024.1365783
Enhanced enrichment of extracellular vesicles for laboratory and clinical research from drop-sized blood samples
Abstract
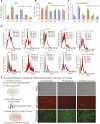
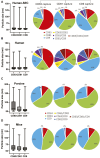
In the realm of biomedical advancement, extracellular vesicles (EVs) are revolutionizing our capacity to diagnose, monitor, and predict disease progression. However, the comprehensive exploration and clinical application of EVs face significant limitations due to the current isolation techniques. The size exclusion chromatography, commercial precipitation reagents, and ultracentrifugation are frequently employed, necessitating skilled operators and entailing challenges related to consistency, reproducibility, quality, and yields. Notably, the formidable challenge of extracellular vesicle isolation persists when dealing with clinical samples of limited availability. This study addresses these challenges by aiming to devise a rapid, user-friendly, and high-recovery EVs isolation technique tailored for blood samples. The NTI-EXO precipitation method demonstrated a 5-fold increase in the recovery of serum EVs compared to current methodologies. Importantly, we illustrate that a mere two drops of blood (∼100 µL) suffice for the recovery of enriched EVs. The integrity and quality of these isolated EVs were rigorously assessed for the size, purity, and contaminants. This method was validated through the successful isolation of EVs from organ transplant recipients to detect disease-specific exosomal markers, including LKB1, SARS-CoV-2 spike protein, and PD-L1. In conclusion, NTI-EXO method can be used for small clinical samples, thereby advancing discoveries in the EV-centric domain and propelling the frontiers of biomedical research and clinical applications.
Keywords: EVS; blood; diagnosis; exosome; marker; transplant.
Copyright © 2024 Guerrero-Alba, Bansal, Sankpal, Mitra, Rahman, Ravichandran, Poulson, Fleming, Smith, Bremner, Mohanakumar and Sankpal.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Bachurski D., Schuldner M., Nguyen P. H., Malz A., Reiners K. S., Grenzi P. C., et al. (2019). Extracellular vesicle measurements with nanoparticle tracking analysis - an accuracy and repeatability comparison between NanoSight NS300 and ZetaView. J. Extracell. Vesicles 8 (1), 1596016. 10.1080/20013078.2019.1596016 - DOI - PMC - PubMed
-
- Bansal S., Limaye A. P., Lee J., Fleming T., Poulson C., Omar A., et al. (2021a). Circulating exosomes induced by respiratory viral infections in lung transplant recipients activate cellular stress, innate immune pathways and epithelial to mesenchymal transition. Transpl. Immunol. 69, 101480. 10.1016/j.trim.2021.101480 - DOI - PubMed
-
- Bansal S., Perincheri S., Fleming T., Poulson C., Tiffany B., Bremner R. M., et al. (2021b). Cutting edge: circulating exosomes with covid spike protein are induced by BNT162b2 (Pfizer-BioNTech) vaccination prior to development of antibodies: a novel mechanism for immune activation by mRNA vaccines. J. Immunol. 207 (10), 2405–2410. 10.4049/jimmunol.2100637 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

