Unraveling the complex interplay: immunopathology and immune evasion strategies of alphaviruses with emphasis on neurological implications
- PMID: 39211797
- PMCID: PMC11358129
- DOI: 10.3389/fcimb.2024.1421571
Unraveling the complex interplay: immunopathology and immune evasion strategies of alphaviruses with emphasis on neurological implications
Abstract
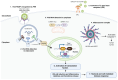
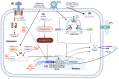
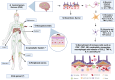
Arthritogenic alphaviruses pose a significant public health concern due to their ability to cause joint inflammation, with emerging evidence of potential neurological consequences. In this review, we examine the immunopathology and immune evasion strategies employed by these viruses, highlighting their complex mechanisms of pathogenesis and neurological implications. We delve into how these viruses manipulate host immune responses, modulate inflammatory pathways, and potentially establish persistent infections. Further, we explore their ability to breach the blood-brain barrier, triggering neurological complications, and how co-infections exacerbate neurological outcomes. This review synthesizes current research to provide a comprehensive overview of the immunopathological mechanisms driving arthritogenic alphavirus infections and their impact on neurological health. By highlighting knowledge gaps, it underscores the need for research to unravel the complexities of virus-host interactions. This deeper understanding is crucial for developing targeted therapies to address both joint and neurological manifestations of these infections.
Keywords: alphavirus; chikungunya; coinfection; immune evasion; immunopathology; mayaro; mouse; nervous system.
Copyright © 2024 de Oliveira Souza, Duarte Júnior, Della Casa, Santoro Rosa, Renia and Claser.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

