Pharmacological Inhibition of N-Acylethanolamine Acid Amidase (NAAA) Mitigates Intestinal Fibrosis Through Modulation of Macrophage Activity
- PMID: 39211986
- PMCID: PMC11836880
- DOI: 10.1093/ecco-jcc/jjae132
Pharmacological Inhibition of N-Acylethanolamine Acid Amidase (NAAA) Mitigates Intestinal Fibrosis Through Modulation of Macrophage Activity
Abstract
Background and aims: Intestinal fibrosis, a frequent complication of inflammatory bowel disease, is characterized by stricture formation with no pharmacological treatment to date. N-acylethanolamine acid amidase (NAAA) is responsible for the hydrolysis of acylethanolamides (AEs, eg, palmitoylethanolamide and oleoylethanolamide). Here, we investigated NAAA and AE signaling in gut fibrosis.
Methods: NAAA and AE signaling were evaluated in human intestinal specimens from patients with stenotic Crohn's disease (CD). Gut fibrosis was induced by 2,4,6-trinitrobenzenesulfonic acid, monitored by colonoscopy, and assessed by qRT-PCR, histological analyses, and confocal microscopy. Immune cells in mesenteric lymph nodes were analyzed by FACS. Colonic fibroblasts were cultured in conditioned media derived from polarized or non-polarized bone marrow-derived macrophages (BMDMs). IL-23 signaling was evaluated by qRT-PCR, ELISA, FACS, and western blot in BMDMs and in lamina propria CX3CR1+ cells.
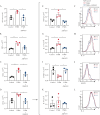
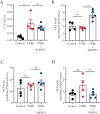
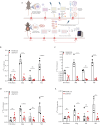
Results: In ileocolonic human CD strictures, increased transcript expression of NAAA was observed with a decrease in its substrates oleoylethanolamide and palmitoylethanolamide. NAAA inhibition reduced intestinal fibrosis in vivo, as indicated by a decrease in inflammatory parameters, collagen deposition, and fibrosis-related genes, including those involved in epithelial-to-mesenchymal transition. More in-depth studies revealed modulation of the immune response related to IL-23 following NAAA inhibition. The antifibrotic actions of NAAA inhibition are mediated by Mφ and M2 macrophages that indirectly affect fibroblast collagenogenesis. NAAA inhibitor AM9053 normalized IL-23 signaling in BMDMs and in lamina propria CX3CR1+ cells.
Conclusions: Our findings provide new insights into the pathophysiological mechanism of intestinal fibrosis and identify NAAA as a promising target for the development of therapeutic treatments to alleviate CD-related fibrosis.
Keywords: Acylethanolamides; IL-23; intestinal fibrosis.
© The Author(s) 2024. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
None declared.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

