Continuation vs Discontinuation of Renin-Angiotensin System Inhibitors Before Major Noncardiac Surgery: The Stop-or-Not Randomized Clinical Trial
- PMID: 39212270
- PMCID: PMC11365013
- DOI: 10.1001/jama.2024.17123
Continuation vs Discontinuation of Renin-Angiotensin System Inhibitors Before Major Noncardiac Surgery: The Stop-or-Not Randomized Clinical Trial
Abstract
Importance: Before surgery, the best strategy for managing patients who are taking renin-angiotensin system inhibitors (RASIs) (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers) is unknown. The lack of evidence leads to conflicting guidelines.
Objective: To evaluate whether a continuation strategy vs a discontinuation strategy of RASIs before major noncardiac surgery results in decreased complications at 28 days after surgery.
Design, setting, and participants: Randomized clinical trial that included patients who were being treated with a RASI for at least 3 months and were scheduled to undergo a major noncardiac surgery between January 2018 and April 2023 at 40 hospitals in France.
Intervention: Patients were randomized to continue use of RASIs (n = 1107) until the day of surgery or to discontinue use of RASIs 48 hours prior to surgery (ie, they would take the last dose 3 days before surgery) (n = 1115).
Main outcomes and measures: The primary outcome was a composite of all-cause mortality and major postoperative complications within 28 days after surgery. The key secondary outcomes were episodes of hypotension during surgery, acute kidney injury, postoperative organ failure, and length of stay in the hospital and intensive care unit during the 28 days after surgery.
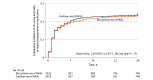
Results: Of the 2222 patients (mean age, 67 years [SD, 10 years]; 65% were male), 46% were being treated with angiotensin-converting enzyme inhibitors at baseline and 54% were being treated with angiotensin receptor blockers. The rate of all-cause mortality and major postoperative complications was 22% (245 of 1115 patients) in the RASI discontinuation group and 22% (247 of 1107 patients) in the RASI continuation group (risk ratio, 1.02 [95% CI, 0.87-1.19]; P = .85). Episodes of hypotension during surgery occurred in 41% of the patients in the RASI discontinuation group and in 54% of the patients in the RASI continuation group (risk ratio, 1.31 [95% CI, 1.19-1.44]). There were no other differences in the trial outcomes.
Conclusions and relevance: Among patients who underwent major noncardiac surgery, a continuation strategy of RASIs before surgery was not associated with a higher rate of postoperative complications than a discontinuation strategy.
Trial registration: ClinicalTrials.gov Identifier: NCT03374449.
Conflict of interest statement
Figures


References
-
- Roshanov PS, Rochwerg B, Patel A, et al. . Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery: an analysis of the vascular events in noncardiac surgery patients cohort evaluation prospective cohort. Anesthesiology. 2017;126(1):16-27. doi:10.1097/ALN.0000000000001404 - DOI - PubMed
-
- Kristensen SD, Knuuti J, Saraste A, et al. ; Authors/Task Force Members . 2014 ESC/ESA guidelines on non-cardiac surgery: cardiovascular assessment and management: the joint task force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383-2431. doi:10.1093/eurheartj/ehu282 - DOI - PubMed
-
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. . 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;130(24):e278-e333. doi:10.1161/CIR.0000000000000106 - DOI - PubMed

