cIMPACT-NOW update 8: Clarifications on molecular risk parameters and recommendations for WHO grading of meningiomas
- PMID: 39212325
- PMCID: PMC11812049
- DOI: 10.1093/neuonc/noae170
cIMPACT-NOW update 8: Clarifications on molecular risk parameters and recommendations for WHO grading of meningiomas
Abstract
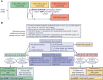
Meningiomas are the most frequent primary intracranial tumors. Hence, they constitute a major share of diagnostic specimens in neuropathology practice. The 2021 WHO Classification of Central Nervous System Tumors ("CNS5") has introduced the first molecular grading parameters for meningioma with oncogenic variants in the TERT promoter and homozygous deletion of CDKN2A/B as markers for CNS WHO grade 3. However, after the publication of the new classification volume, clarifications were requested, not only on novel but also on long-standing questions in meningioma grading that were beyond the scope of the WHO "blue book." In addition, more recent research into possible new molecular grading parameters could not yet be implemented in the 2021 classification but constitutes a compelling body of literature. Hence, the consortium to inform molecular and practical approaches to CNS tumor taxonomy-not official WHO (cIMPACT-NOW) Steering Committee convened a working group to provide such clarification and assess the evidence of possible novel molecular criteria. As a result, this cIMPACT-NOW update provides guidance for more standardized morphological evaluation and interpretation, most prominently pertaining to brain invasion, identifies scenarios in which advanced molecular testing is recommended, proposes to assign CNS WHO grade 2 for cases with CNS WHO grade 1 morphology but chromosomal arm 1p deletion in combination with 22q deletion and/or NF2 oncogenic variants, and discusses areas in which the current evidence is not yet sufficient to result in new recommendations.
Keywords: WHO classification; cIMPACT-NOW; classification; grading; meningioma; molecular neuropathology.
Published by Oxford University Press on behalf of the Society for Neuro-Oncology 2024.
Conflict of interest statement
A.v.D. reports being co-founder and shareholder of Heidelberg Epignostix GmbH, royalties for IDH1 R132H mutation-specific antibody from DIANOVA GmbH, and royalties for BRAF V600E mutation-specific antibody from Roche Ventana. D.C. reports being co-founder and shareholder of Heidelberg Epignostix GmbH, royalties for IDH1 R132H mutation-specific antibody from DIANOVA GmbH, and royalties for BRAF V600E mutation-specific antibody from Roche Ventana, and research funding from NOVOCURE. D.R.R. reports honoraria for consulting or lectures from Gamma Tile, Tipping Point, Nerviano Medical Science, Boston Scientific, Duke University, Memorial Sloan Kettering Cancer Center, St Jude, and serves as SNO Radiation Track Co-Chair. F.S. reports being co-founder and shareholder of Heidelberg Epignostix GmbH, and patents or patent applications for classification of cancer and methylation-based classification, honoraria from Bayer, Roche and Illumina, speaker support from Agilent. G.R. serves on the data safety board of HIT HGG trial. KDA reports patents or patent applications for DNA methylation-based cancer diagnostics for tumors of the central nervous system, kidney and hematopoietic system, and for predicting DNA methylation and tumor types from histopathology. M.R.G. reports honoraria from George Washington University and serves on the Advisory Board for the ARISTOCRAT Trial, and as grant reviewer and advisor for the Brain Tumour Charity. P.K.B. reports grants for institution from Breast Cancer Research Foundation, Eli Lilly, Merck, Kinnate, MGH Research Scholar Award, American Association for Cancer Research, Melanoma Research Alliance, Krantz, Mirati, and consulting fees from Advise Connect Inspire Atavistik Bio, Axiom, CraniUS, Genentech, InCephalo, Kazia, Medscape, MPM Capital Advisors, Sintetica, honoraria from Genentech, MPM Capital Advisors, serves on the monitoring or advisory boards of Kazia, N2M2, CraniUS, and as ASCO co-chair multisite guidelines committee, SNO Medical Oncology Representative, and received other support from Amgen, Eli Lilly, AstraZeneca, Merck, Bristol Myers Squibb, Genentech-Roche, GSK, Kazia, PfizerAlliance Neuro-Oncology Committee Co-Chair. P.W. reports lecture honoraria from Chimerix. S.D. serves as Siteman Cancer Center Protocol Review and Monitoring Committee member and Chair of the Constitution Committee, American Association of Neuropathologists. S.S., D.N.L., G.Z., A.P., D.J.B., and C.S. report no disclosures.
Figures

References
-
- Perry A, Stafford SL, Scheithauer BW, Suman VJ, Lohse CM.. Meningioma grading: an analysis of histologic parameters. Am J Surg Pathol. 1997;21(12):1455–1465. - PubMed
-
- Perry A, Scheithauer BW, Stafford SL, Lohse CM, Wollan PC.. “Malignancy” in meningiomas: a clinicopathologic study of 116 patients, with grading implications. Cancer. 1999;85(9):2046–2056. - PubMed
-
- Biczok A, Jungk C, Egensperger R, et al. Microscopic brain invasion in meningiomas previously classified as WHO grade I is not associated with patient outcome. J Neurooncol. 2019;145(3):469–477. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

