Colchicine prevents accelerated atherosclerosis in TET2-mutant clonal haematopoiesis
- PMID: 39212933
- PMCID: PMC11560281
- DOI: 10.1093/eurheartj/ehae546
Colchicine prevents accelerated atherosclerosis in TET2-mutant clonal haematopoiesis
Abstract
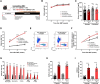
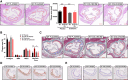
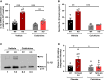
Background and aims: Somatic mutations in the TET2 gene that lead to clonal haematopoiesis (CH) are associated with accelerated atherosclerosis development in mice and a higher risk of atherosclerotic disease in humans. Mechanistically, these observations have been linked to exacerbated vascular inflammation. This study aimed to evaluate whether colchicine, a widely available and inexpensive anti-inflammatory drug, prevents the accelerated atherosclerosis associated with TET2-mutant CH.
Methods: In mice, TET2-mutant CH was modelled using bone marrow transplantations in atherosclerosis-prone Ldlr-/- mice. Haematopoietic chimeras carrying initially 10% Tet2-/- haematopoietic cells were fed a high-cholesterol diet and treated with colchicine or placebo. In humans, whole-exome sequencing data and clinical data from 37 181 participants in the Mass General Brigham Biobank and 437 236 participants in the UK Biobank were analysed to examine the potential modifying effect of colchicine prescription on the relationship between CH and myocardial infarction.
Results: Colchicine prevented accelerated atherosclerosis development in the mouse model of TET2-mutant CH, in parallel with suppression of interleukin-1β overproduction in conditions of TET2 loss of function. In humans, patients who were prescribed colchicine had attenuated associations between TET2 mutations and myocardial infarction. This interaction was not observed for other mutated genes.
Conclusions: These results highlight the potential value of colchicine to mitigate the higher cardiovascular risk of carriers of somatic TET2 mutations in blood cells. These observations set the basis for the development of clinical trials that evaluate the efficacy of precision medicine approaches tailored to the effects of specific mutations linked to CH.
Keywords: Atherosclerosis; CHIP; Colchicine; Inflammation; Precision medicine; TET2.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures

Comment in
-
Clonal haematopoiesis: an emerging causal risk factor for atherosclerotic CVD.Nat Rev Cardiol. 2024 Nov;21(11):741. doi: 10.1038/s41569-024-01081-3. Nat Rev Cardiol. 2024. PMID: 39289541 No abstract available.
References
MeSH terms
Substances
Grants and funding
- K99HG012956/HG/NHGRI NIH HHS/United States
- FPU18/02913/ESF Investing in your future
- Ministerio de Ciencia, Innovación y Universidades
- Instituto de Salud Carlos III
- TNE-18CVD04/Leducq Foundation
- K99HL165024/U.S. National Heart, Lung, and Blood Institute
- 940166/American Heart Association
- K99 HG012956/HG/NHGRI NIH HHS/United States
- Japan Society for the Promotion of Science
- Pro CNIC Foundation
- European Union NextGenerationEU
- LCF/PR/HR17/52150007/'la Caixa
- AEI
- MICIN
- K08 HL166687/HL/NHLBI NIH HHS/United States
- FJC2020-042841-I/MICIU
- 'La Marató TV3'
- CEX2020-001041-S/Severo Ochoa Center of Excellence
- R00 HG012956/HG/NHGRI NIH HHS/United States
- PLEC2021-008194/ESF Investing in your future
- PID2021-126580OB-I00/PRTR
- 202314-31/ERDF/EU
- R01 HL148050/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

