Effect of Dapagliflozin on Accelerometer-Based Measures of Physical Activity in Patients With Heart Failure: An Analysis of the DETERMINE Trials
- PMID: 39212948
- PMCID: PMC11472896
- DOI: 10.1161/CIRCHEARTFAILURE.124.012349
Effect of Dapagliflozin on Accelerometer-Based Measures of Physical Activity in Patients With Heart Failure: An Analysis of the DETERMINE Trials
Abstract
Background: Wearable accelerometers can quantify the frequency and intensity of physical activity during everyday life and may provide complementary data to established functional outcome measures on the effect of heart failure therapies on functional limitations.
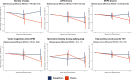
Methods: In a voluntary substudy of the DETERMINE trials (Dapagliflozin Effect on Exercise Capacity Using a 6-Minute Walk Test in Patients With Heart Failure), patients wore a waist-worn triaxial accelerometer for as long as possible (ideally for 24 h/d for 7 days) at 3 points during the trial, between the screening visit and randomization (baseline data), and during weeks 8 and 14 to 16. Accelerometer outcomes included the change from baseline to week 16 in the total number of steps, time spent in light-to-vigorous physical activity, time spent in moderate-to-vigorous physical activity, movement intensity during walking, number of vector magnitude units' and total activity counts.
Results: Adequate baseline and week 16 accelerometer data were available for 211 of 817 (26%) randomized patients (defined as ≥10 hours of wear time for ≥3 days). Dapagliflozin had a favorable effect on the mean change from baseline at 16 weeks in the number of steps (between-group difference, 778 [95% CI, 240-1315]), time spent in moderate-to-vigorous physical activity (0.16 [95% CI, 0.03-0.29] hours), and in the mean vector magnitude units (25 [95% CI, 0.1-49] counts per minute). There were no between-group differences in the other accelerometer outcomes of interest.
Conclusions: In this exploratory analysis of the DETERMINE trials, dapagliflozin had a beneficial effect on selected accelerometer-based measures of physical activity in patients with heart failure across the entire left ventricular ejection fraction spectrum, yet did not improve 6-minute walk distance, as previously reported. These data suggest that accelerometer-based measurements of everyday activity may provide complementary information to 6-minute walk distance and identify beneficial effects of treatment not detected by 6-minute walk distance.
Registration: URL: https://www.clinicaltrials.gov; Unique identifiers: NCT03877237 and NCT03877224.
Keywords: accelerometry; exercise; sodium-glucose transporter 2 inhibitors; walk test; wearable electronic devices.
Conflict of interest statement
Dr Docherty’s employer, the University of Glasgow, has been remunerated by AstraZeneca for his work on clinical trials; he has received speaker fees from AstraZeneca, Boehringer Ingelheim, Pharmacosmos, Translational Medical Academy, and Radcliffe Cardiology; has served on Advisory Boards for Us2.ai and holds stock in the company; has served on an advisory board and served on a clinical end point committee for Bayer AG; has performed consultancy for FIRE-1; and has received research grant support (paid to his institution) from AstraZeneca, Roche, Novartis, and Boehringer Ingelheim. Drs Buendia Lopez, Cowie, Hammarstedt, Langkilde, and Reicher and F. Folkvaljon are employees of AstraZeneca and may own stock or stock options. The institution of Dr de Boer has received research grants or fees from AstraZeneca, Abbott, Bristol Myers Squibb, Cardior Pharmaceuticals GmbH, NovoNordisk, and Roche; he has had speaker engagements with or received fees from or served on an advisory board for Abbott, AstraZeneca, Bristol Myers Squibb, Cardior Pharmaceuticals GmbH, NovoNordisk, and Roche; and has received travel support from Abbott, Cardior Pharmaceuticals GmbH, and Novo Nordisk. Dr Kitzman has been a consultant for AstraZeneca, Pfizer, Corvia Medical, Bayer, Boehringer Ingelheim, Novo Nordisk, Rivus, and St. Luke’s Medical Center; has received grant support from Novartis, AstraZeneca, Bayer, Pfizer, Novo Nordisk, Rivus, and St. Luke’s Medical Center; and owns stock in Gilead Sciences. Dr Kosiborod has served as a consultant or on an advisory board for 35Pharma, Alnylam, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Dexcom, Eli Lilly, Esperion Therapeutics, Imbria, Janssen, Lexicon Pharmaceuticals, Merck (Diabetes and Cardiovascular), Novo Nordisk, Pharmacosmos, Pfizer, Sanofi, scPharmaceuticals, Structure Therapeutics, Vifor, and Youngene Therapeutics; has received research grants from AstraZeneca, Boehringer Ingelheim, and Pfizer; holds stocks in Artera Health and Saghmos Therapeutics; has received honoraria from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk; and has received other research support from AstraZeneca. Dr Senni has received honoraria or consulting fees from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Merck, MSD, Novartis, Novo Nordisk, and Vifor. Dr Shah was supported by research grants from the US National Institutes of Health (NIH; U54 HL160273; R01 HL140731; and R01 HL149423); has received research funding from AstraZeneca, Corvia, and Pfizer; and has received consulting fees from Abbott, Alleviant, Amgen, Aria CV, AstraZeneca, Axon Therapies, Bayer, Boehringer Ingelheim, Boston Scientific, BridgeBio, Bristol Myers Squibb, Corvia, Cytokinetics, Edwards Lifesciences, Eli Lilly, Eidos, Imara, Impulse Dynamics, Intellia, Ionis, Merck, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Pfizer, Prothena, Regeneron, Rivus, Sardocor, Shifamed, Tenax, Tenaya, and Ultromics. Dr Verma holds a Tier 1 Canada Research Chair in Cardiovascular Surgery and reports receiving grants or research support or speaking honoraria from Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Canadian Medical and Surgical Knowledge Translation Research Group, Eli Lilly, HLS Therapeutics, Humber River Health, Janssen, Merck, Novartis, Novo Nordisk, Pfizer, PhaseBio, S&L Solutions Event Management Inc, and Sanofi. Dr Solomon has received research grants from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, Bristol Myers Squibb, Celladon, Cytokinetics, Eidos, Gilead, GlaxoSmithKline, Ionis, Lilly, Mesoblast, MyoKardia, NIH/National Heart‚ Lung‚ and Blood Institute (NHLBI), Neurotronik, Novartis, NovoNordisk, Respicardia, Sanofi Pasteur, Theracos, and Us2.ai and has consulted for Abbott, Action, Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi Sankyo, GlaxoSmithKline, Lilly, Merck, Myokardia, Novartis, Roche, Theracos, Quantum Genomics, Cardurion, Janssen, Cardiac Dimensions, Tenaya, Sanofi Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, American Regent, and Sarepta. Dr McMurray reports receiving support from British Heart Foundation Centre of Research Excellence Grant RE/18/6/34217 and the Vera Melrose Heart Failure Research Fund; payments through Glasgow University from work on clinical trials, consulting, and grants from Amgen, AstraZeneca, Bayer, Cardurion, Cytokinetics, GlaxoSmithKline and Novartis, British Heart Foundation, NIH/NHLBI, Boehringer Ingelheim, SQ Innovations, and Catalyze Group; personal consultancy fees from Alynylam Pharmaceuticals, Amgen, AnaCardio, AstraZeneca, Bayer, Berlin Cures, Bristol Myers Squibb, Cardurion, Cytokinetics, Ionis Pharmaceuticals, Novartis, Regeneron Pharmaceuticals, and River 2 Renal Corp; personal lecture fees from Abbott, Alkem Metabolics, Astra Zeneca, Blue Ocean Scientific Solutions Ltd, Boehringer Ingelheim, Canadian Medical and Surgical Knowledge, Emcure Pharmaceuticals Ltd, Eris Lifesciences, European Academy of CME, Hikma Pharmaceuticals, Imagica Health, Intas Pharmaceuticals, J.B. Chemicals and Pharmaceuticals Ltd, Lupin Pharmaceuticals, Medscape/Heart.Org, ProAdWise Communications, Radcliffe Cardiology, Sun Pharmaceuticals, The Corpus, Translation Research Group, and Translational Medicine Academy; and on the Data Safety Monitoring Board for WIRB-Copernicus Group Clinical Inc. He is a director of Global Clinical Trial Partners Ltd.
Figures
References
-
- O’Donnell J, Smith-Byrne K, Velardo C, Conrad N, Salimi-Khorshidi G, Doherty A, Dwyer T, Tarassenko L, Rahimi K. Self-reported and objectively measured physical activity in people with and without chronic heart failure: UK Biobank analysis. Open Heart. 2020;7:e001099. doi: 10.1136/openhrt-2019-001099 - PMC - PubMed
-
- Butt JH, Docherty KF, Kosiborod MN, Inzucchi SE, Køber L, Langkilde AM, Martinez FA, Bengtsson O, Ponikowski P, Sabatine MS, et al. . Dapagliflozin and physical and social activity limitations in heart failure with reduced ejection fraction. JACC Heart Fail. 2023;11:1411–1423. doi: 10.1016/j.jchf.2023.04.016 - PubMed
-
- Psotka MA, Abraham WT, Fiuzat M, Filippatos G, Lindenfeld J, Ahmad T, Felker GM, Jacob R, Kitzman DW, Leifer ES, et al. . Functional and symptomatic clinical trial endpoints: the HFC-ARC scientific expert panel. JACC Heart Fail. 2022;10:889–901. doi: 10.1016/j.jchf.2022.09.012 - PubMed
-
- Piepoli MF, Hussain RI, Comin-Colet J, Dosantos R, Ferber P, Jaarsma T, Edelmann F. OUTSTEP-HF: randomised controlled trial comparing short-term effects of sacubitril/valsartan versus enalapril on daily physical activity in patients with chronic heart failure with reduced ejection fraction. Eur J Heart Fail. 2021;23:127–135. doi: 10.1002/ejhf.2076 - PubMed
-
- Pieske B, Wachter R, Shah SJ, Baldridge A, Szeczoedy P, Ibram G, Shi V, Zhao Z, Cowie MR; PARALLAX Investigators and Committee members. Effect of sacubitril/valsartan vs standard medical therapies on plasma NT-proBNP concentration and submaximal exercise capacity in patients with heart failure and preserved ejection fraction: the PARALLAX randomized clinical trial. JAMA. 2021;326:1919–1929. doi: 10.1001/jama.2021.18463 - PMC - PubMed

