The Profile of the Italian Centers for Cognitive Disorders and Dementia in the Context of New Drugs in Alzheimer's Disease
- PMID: 39213073
- PMCID: PMC11492016
- DOI: 10.3233/JAD-240594
The Profile of the Italian Centers for Cognitive Disorders and Dementia in the Context of New Drugs in Alzheimer's Disease
Abstract
Background: The wait for the upcoming disease-modifying therapies (DMT) for Alzheimer's disease in Europe is raising questions about the preparedness of national healthcare systems to conduct accurate diagnoses and effective prescriptions. In this article, we focus on the current situation in Italy.
Objective: The primary goal is to propose a profile of the Italian Centers for Cognitive Disorders and Dementias (CCDDs) that could be taken into consideration by regional and autonomous provincial authorities when deciding on the prescribing centers for DMT.
Methods: Based on responses to a national survey on CCDDs in Italy, we identified the CCDDs that meet the requirements for effective prescription: 1) Multidisciplinary team; 2) Minimum Core Test for the neuropsychological assessment; 3) PET, CSF, and Brain MRI assessments. Univariate and multivariate comparisons were conducted between CCDDs that met the criteria and the others.
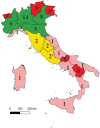
Results: Only 10.4% of CCDDs met the requirements for effective DMT prescription, mainly located in Northern Italy. They are also characterized by longer opening hours, a higher number of professionals, a university location, and a higher frequency of conducting genetic tests, and could potentially result in prescribing centers.
Conclusions: The findings suggest that the Italian national healthcare system may benefit from further enhancements to facilitate the effective prescription of DMTs. This could involve initiatives to reduce fragmentation, ensure adequate resources and equipment, and secure sufficient funding to support this aspect of healthcare delivery.
Keywords: public health; Alzheimer’s disease; Centers for Cognitive Disorders and Dementias; cognitive assessment; disease-modifying therapy; memory clinic.
Conflict of interest statement
The authors have no conflict of interest to report.
Figures
References
-
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Dement 2023; 19: 1598–1695. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

